Question
5. (8 pts) The Hydrogen Atom. You will need Table 14.2 to complete the following problems. (a) Calculate the probability that a 1s electron
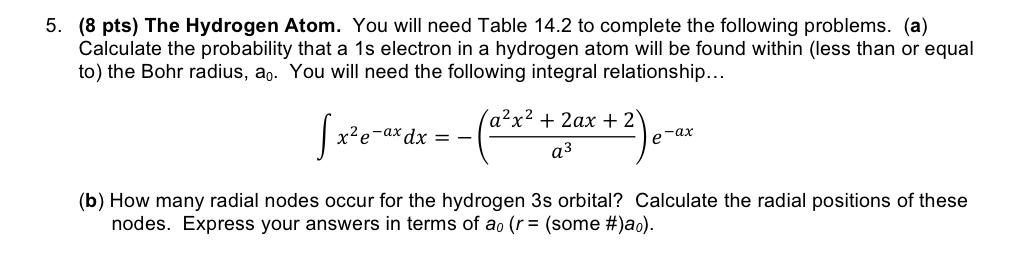
5. (8 pts) The Hydrogen Atom. You will need Table 14.2 to complete the following problems. (a) Calculate the probability that a 1s electron in a hydrogen atom will be found within (less than or equal to) the Bohr radius, ao. You will need the following integral relationship... ax2 +2ax + 2 xe-a* -ax dx = - e-ax a3 (b) How many radial nodes occur for the hydrogen 3s orbital? Calculate the radial positions of these nodes. Express your answers in terms of ao (r = (some #)ao).
Step by Step Solution
3.29 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
a The groundstate 1s orbital wave function for hydrogen atom is 1s 1a 0 3 e r a 0 The ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Peter Atkins
7th Edition
978-0716735397, 716735393, 978-0716743880
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



