Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. a) Sketch the energy vs. distance curve, U(r) for a covalent bond. Directly beneath the potential energy curve, sketch the corresponding force (F)
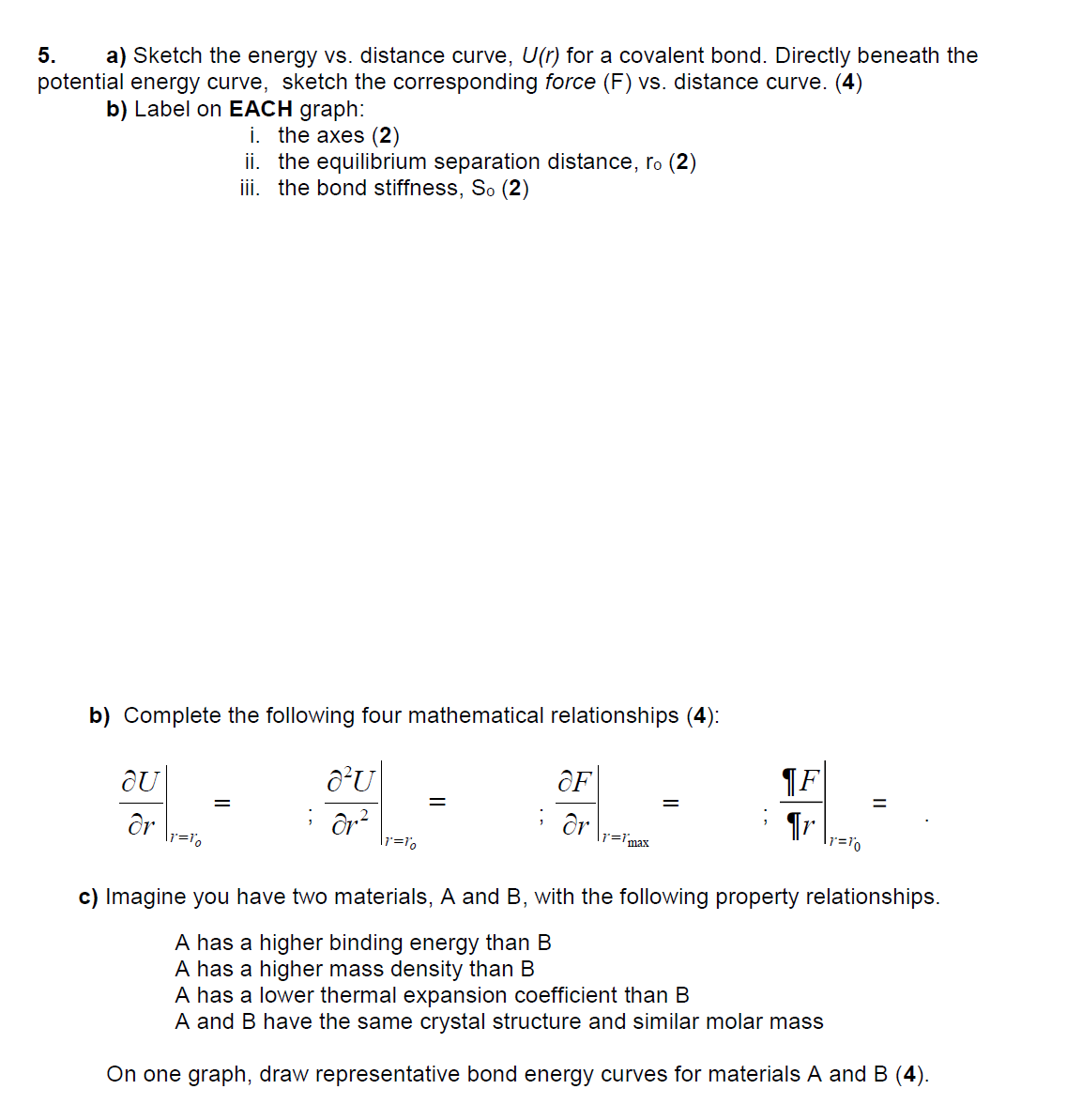
5. a) Sketch the energy vs. distance curve, U(r) for a covalent bond. Directly beneath the potential energy curve, sketch the corresponding force (F) vs. distance curve. (4) b) Label on EACH graph: i. the axes (2) ii. the equilibrium separation distance, ro (2) iii. the bond stiffness, So (2) b) Complete the following four mathematical relationships (4): au JU dr = OF dr |1='max F =10 dr =1 =10 c) Imagine you have two materials, A and B, with the following property relationships. A has a higher binding energy than B A has a higher mass density than B A has a lower thermal expansion coefficient than B A and B have the same crystal structure and similar molar mass On one graph, draw representative bond energy curves for materials A and B (4).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


