Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. Answer ALL parts. a) Given that a compound of molecular formula CH12O2 recorded a max of 215 nm, propose TWO different possible structures
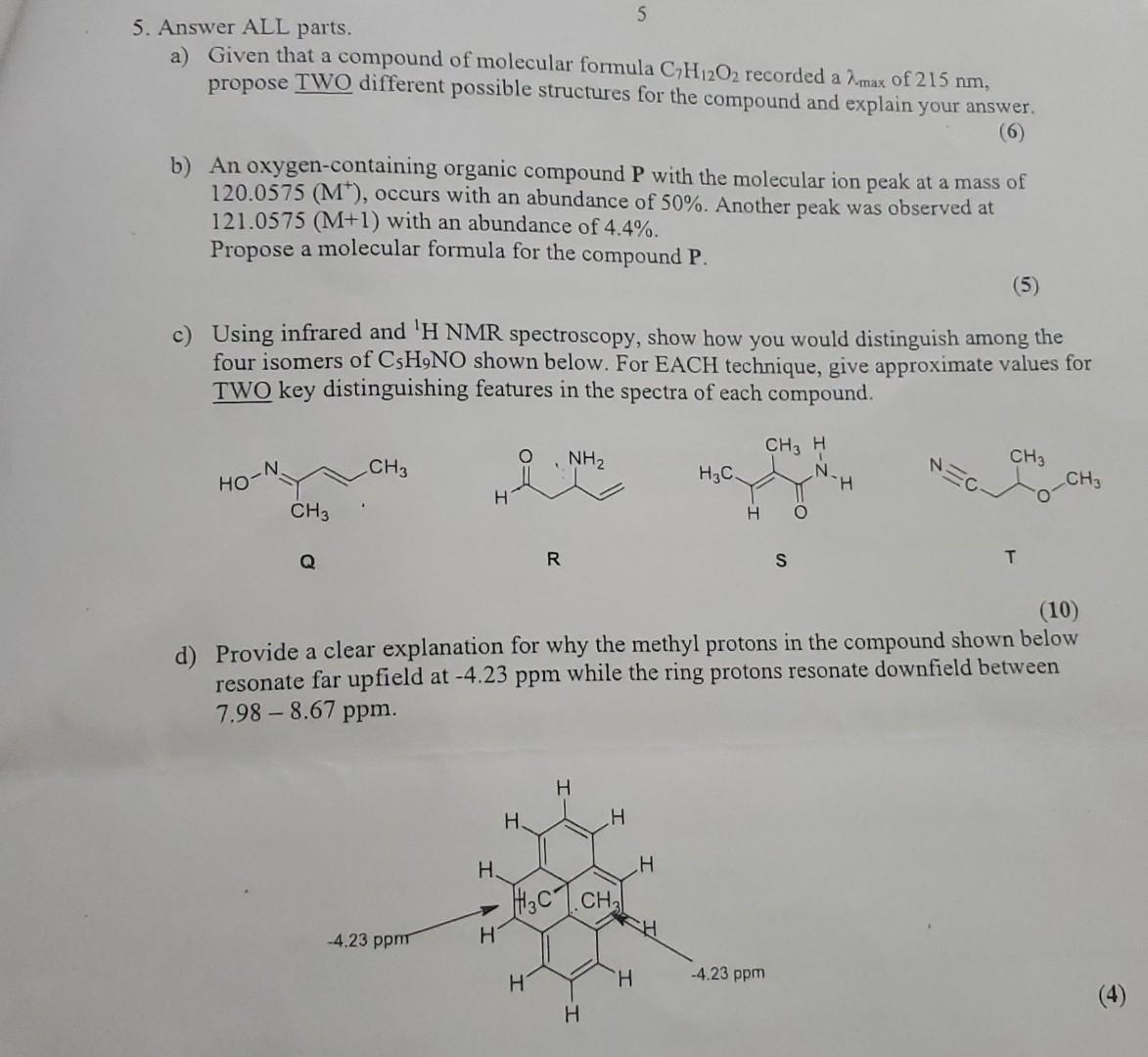
5. Answer ALL parts. a) Given that a compound of molecular formula CH12O2 recorded a max of 215 nm, propose TWO different possible structures for the compound and explain your answer. (6) b) An oxygen-containing organic compound P with the molecular ion peak at a mass of 120.0575 (M), occurs with an abundance of 50%. Another peak was observed at 121.0575 (M+1) with an abundance of 4.4%. Propose a molecular formula for the compound P. (5) Using infrared and 'H NMR spectroscopy, show how you would distinguish among the four isomers of CsH9NO shown below. For EACH technique, give approximate values for TWO key distinguishing features in the spectra of each compound. HO CH3 Q CH3 -4.23 ppm H. H H. R NH H H 5 H3C.CH H H (10) d) Provide a clear explanation for why the methyl protons in the compound shown below resonate far upfield at -4.23 ppm while the ring protons resonate downfield between 7.98-8.67 ppm. H H H3C- H CH3 H N H O S -4.23 ppm H CH3 T CH3
Step by Step Solution
★★★★★
3.48 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Based on the image youve provided you have a series of questions related to organic chemistry specifically related to structure determination and spectroscopy Lets address each part a For the molecula...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


