Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. Calculate the height of a water column if the two glass plates in Q4 formed an angle of 10 at the water surface.
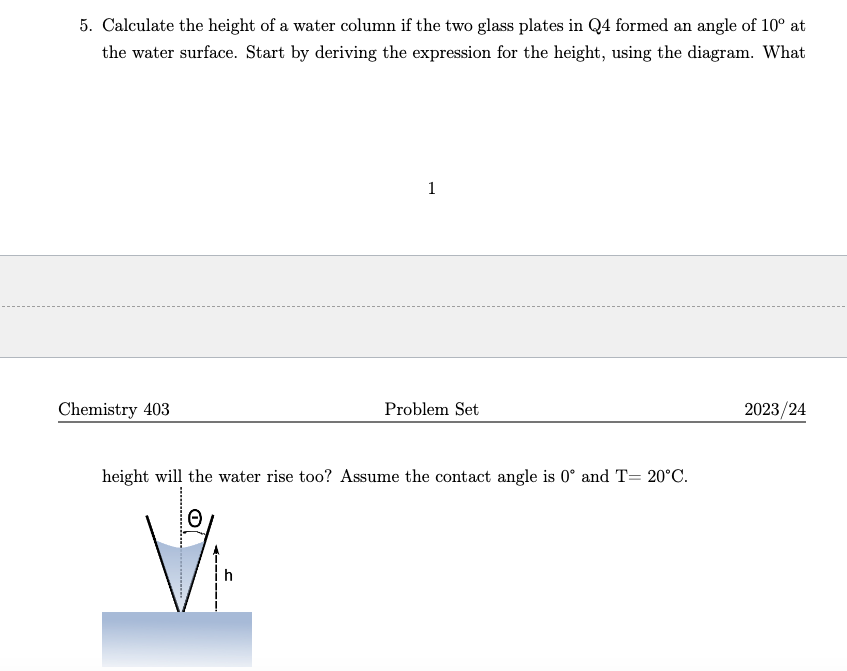


5. Calculate the height of a water column if the two glass plates in Q4 formed an angle of 10 at the water surface. Start by deriving the expression for the height, using the diagram. What Chemistry 403 1 Problem Set height will the water rise too? Assume the contact angle is 0 and T= 20C. 2023/24 4. Capillarity: (a) Calculate the height of a water column between two glass plates separated by 0.05mm at 20C. Assume the plates are 5 cm in width and the contact angle is 0. Start by deriving the capillary rise equation for this geometry neglecting end effects. (b) Estimate the amount of reversible work that would need to be done to pull these plates apart, assuming that the glass remains water wet after they are separated. Make sure to use a few simplifying assumptions in your calculation. (c) the number of moles per unit area (mol m) for this film. 3. The surface tension of water is 72.8 and 58.0 mN m-1 at 20 and 95C respectively. The density of water is 0.988 and 0.958 g cm at 20 and 95C respectively. Calculate the height of the water column at these two temperatures for tubes with internal radii of (a) 1.0 mm, (b) 0.025 mm. 4. Capillarity:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


