Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5 practice questions I don't understand please help today thank you thank you (8.4)What are the products of the following reaction? Al(s) + HSO4(aq) Al(SO4)3(aq)
5 practice questions I don't understand please help today thank you thank you

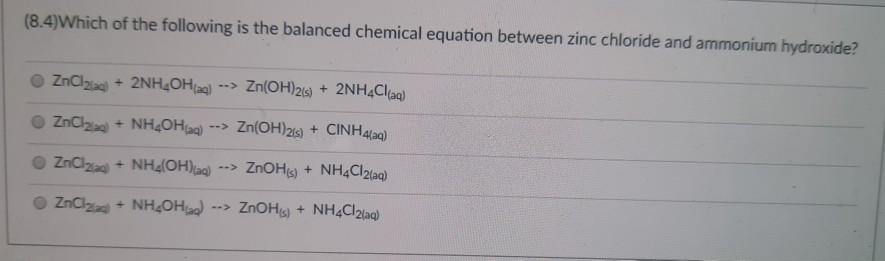
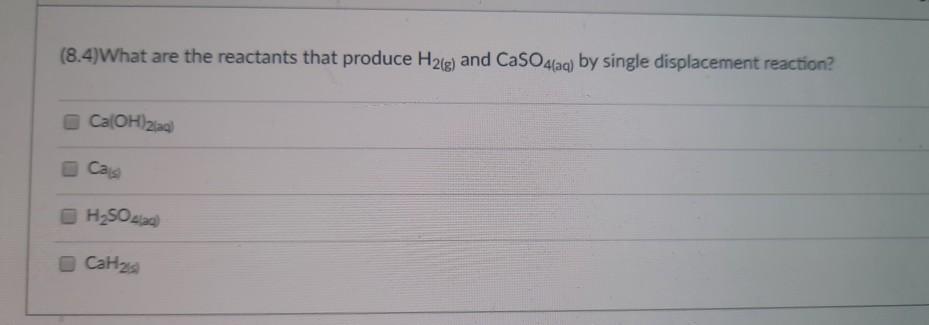
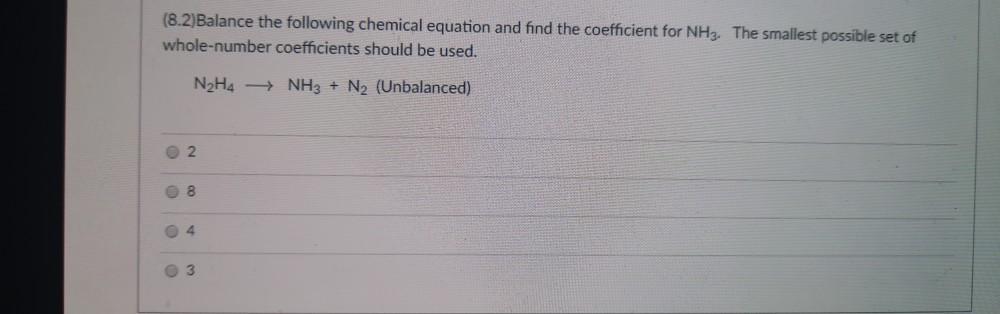
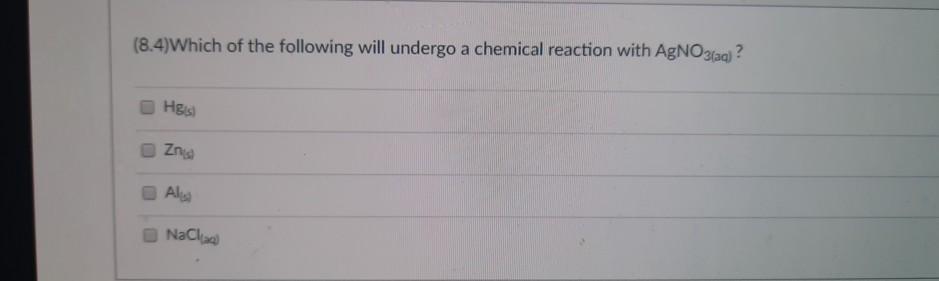
(8.4)What are the products of the following reaction? Al(s) + HSO4(aq) Al(SO4)3(aq) H(g) 2H(g) AISO4(aq) HS AlH3(aq) (8.4) Which of the following is the balanced chemical equation between zinc chloride and ammonium hydroxide? ZnCl + 2NHOH(aq) --> Zn(OH)2(s) + 2NH4Cl(aq) ZnCl + NHOH(aq) --> Zn(OH)2(s) + CINH4(aq) ZnCl + NH4(OH)(aq) --> ZnOH(s) + NH4Cl2(aq) ZnCl + NH4OH) ZnOH(s) + NH4Cl2(aq) (8.4) What are the reactants that produce H2(g) and CaSO4(aq) by single displacement reaction? Ca(OH)2(aq) Casi HSO4(ad) CaH (8.2)Balance the following chemical equation and find the coefficient for NH3. The smallest possible set of whole-number coefficients should be used. NH4NH3 + N (Unbalanced) 2 08 04 3 (8.4)Which of the following will undergo a chemical reaction with AgNO3(aq)? H&is) Zn Al NaCl)
Step by Step Solution
★★★★★
3.37 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


