Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(50%) Problem 1: Large helium-filled balloons are used to lift scientific equipment to high altitudes. 50% Part (a) What is the pressure, in atmospheres,
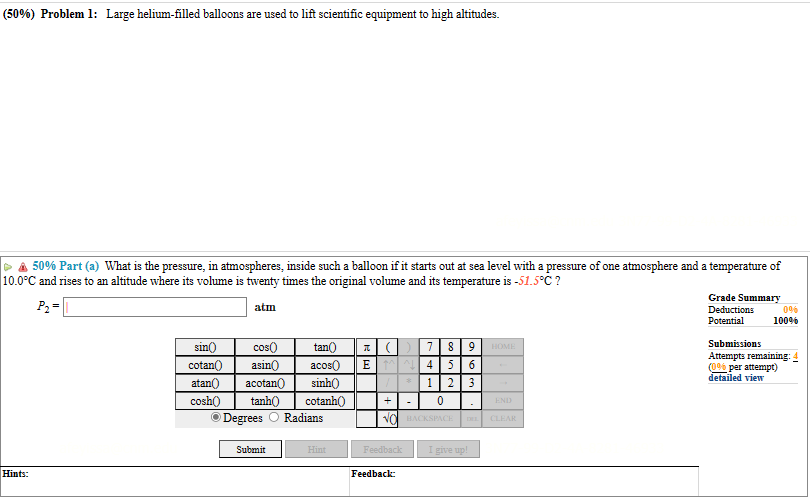
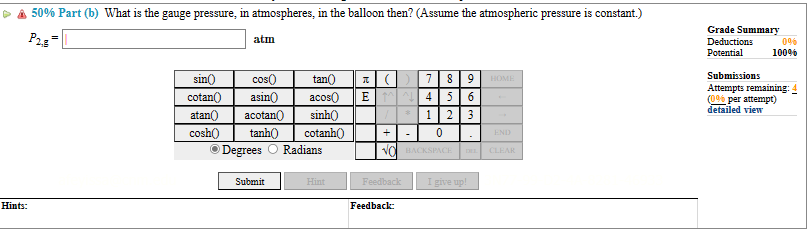
(50%) Problem 1: Large helium-filled balloons are used to lift scientific equipment to high altitudes. 50% Part (a) What is the pressure, in atmospheres, inside such a balloon if it starts out at sea level with a pressure of one atmosphere and a temperature of 10.0C and rises to an altitude where its volume is twenty times the original volume and its temperature is -51.5C? Hints: P- atm sin( cos( tan() cotan() asin acos (7 EMN 8 9 HOME 4 5 6 atan() acotan cosh() tanh() sinh cotanh() 1 2 3 + - 0 END Degrees Radians NO BACKSPACE DEL CLEAR Submit Hint Feedback I give up! Feedback: Grade Summary Deductions Potential Submissions 0% 100% Attempts remaining: (0% per attempt) detailed view Hints: 50% Part (b) What is the gauge pressure, in atmospheres, in the balloon then? (Assume the atmospheric pressure is constant.) P2,8 atm Grade Summary Deductions Potential 0% 100% Submissions Attempts remaining: (0% per attempt) detailed view sin() cotan() cos tan() asin acos () 7 4 5 8 9 HOME 6 atan() acotan sinh() 1 2 3 cosh() tanh() cotanh() + - 0 END Degrees Radians NO BACKSPACE DEL CLEAR Submit Hint Feedback I give up! Feedback:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


