Question
50.0 cm of dilute hydrochloric acid, concentration 1.00 mol dm of dilute sodium hydroxide solution, concentration 1.00 mol dm A temperature rise of 6.5
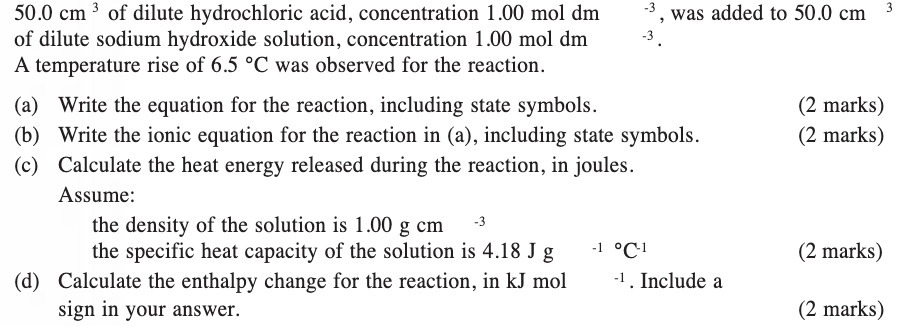
50.0 cm of dilute hydrochloric acid, concentration 1.00 mol dm of dilute sodium hydroxide solution, concentration 1.00 mol dm A temperature rise of 6.5 C was observed for the reaction. -3 -3. the density of the solution is 1.00 g cm -3 the specific heat capacity of the solution is 4.18 Jg (d) Calculate the enthalpy change for the reaction, in kJ mol sign in your answer. (a) Write the equation for the reaction, including state symbols. (b) Write the ionic equation for the reaction in (a), including state symbols. (c) Calculate the heat energy released during the reaction, in joules. Assume: was added to 50.0 cm -1 C- -1. Include a (2 marks) (2 marks) (2 marks) 3 (2 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Basic Technical Mathematics
Authors: Allyn J. Washington, Richard Evans
12th Edition
0137529899, 9780137529896
Students also viewed these Algorithms questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



