Question: 510. Definitions for a multicomponent flash are as follows: x, yi liquid and vapor mole fractions, respectively = feed mole fraction Zj K =y/x-equilibrium
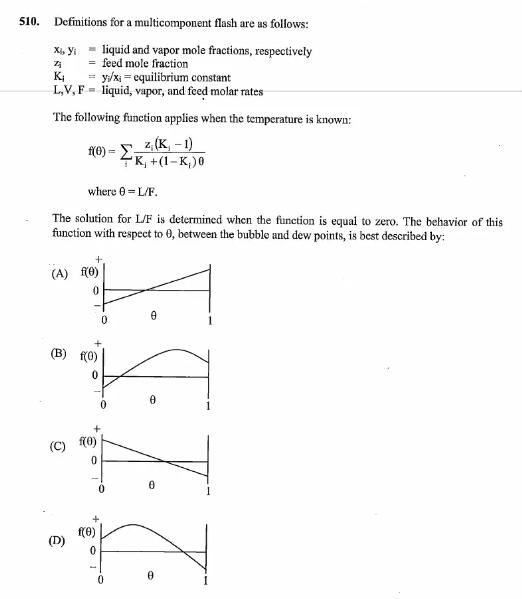
510. Definitions for a multicomponent flash are as follows: x, yi liquid and vapor mole fractions, respectively = feed mole fraction Zj K =y/x-equilibrium constant L,V, F liquid, vapor, and feed molar rates The following function applies when the temperature is known: f(0)-- = z (K-1) +K, +(1-K)0 where 0=L/F. The solution for L/F is determined when the function is equal to zero. The behavior of this function with respect to 0, between the bubble and dew points, is best described by: (A) f(0) 0 + (B) f(0) 0 (C) (0) 0 0 (D) f(0) 0 +
Step by Step Solution
There are 3 Steps involved in it
To solve this problem we need to understand the behavior of the given function f f with respect to w... View full answer

Get step-by-step solutions from verified subject matter experts


