Answered step by step
Verified Expert Solution
Question
1 Approved Answer
51-53. Silver chloride is a relatively insoluble salt. Only 1.92 mg of AgCl will 3 points dissolve per liter of water at 250C. What concentration
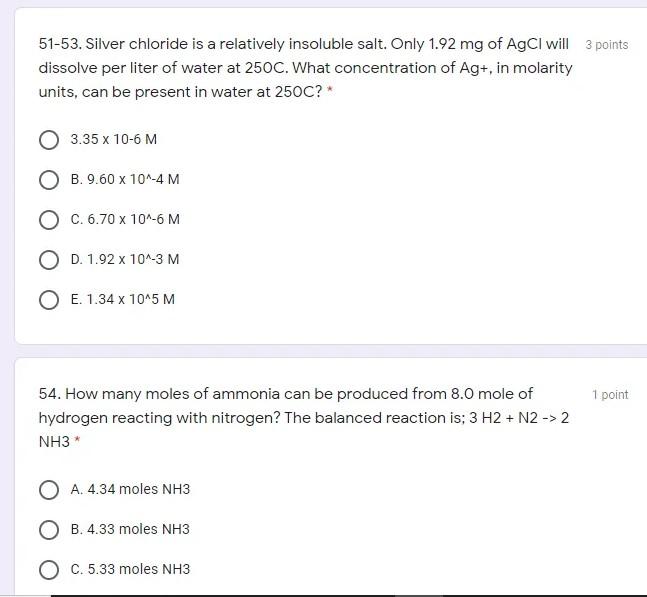
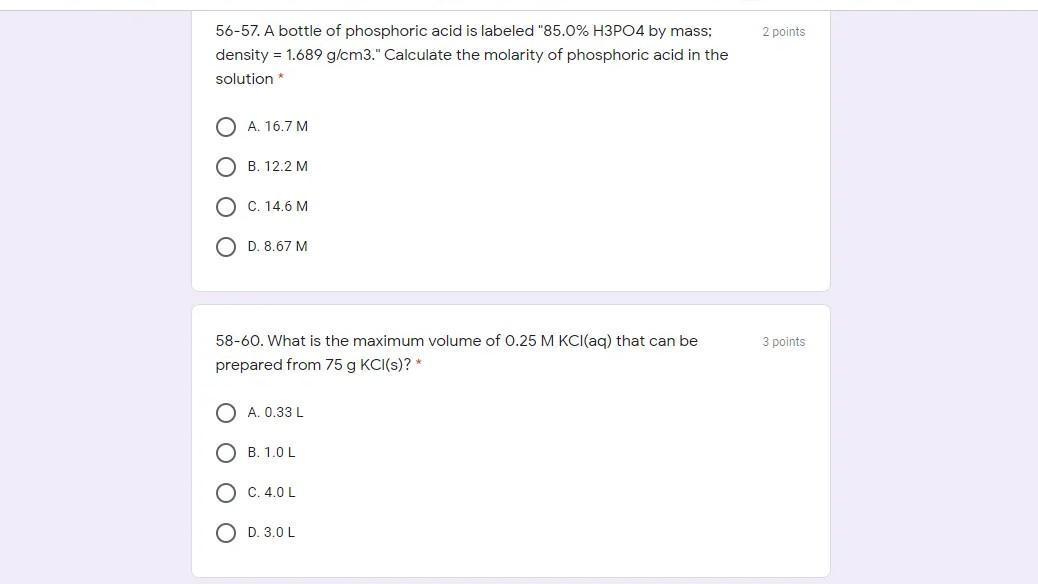
51-53. Silver chloride is a relatively insoluble salt. Only 1.92 mg of AgCl will 3 points dissolve per liter of water at 250C. What concentration of Ag+, in molarity units, can be present in water at 250C?* 3.35 x 10-6 M B. 9.60 x 10^-4 M C. 6.70 x 10^-6 M O D. 1.92 x 10^-3 M O E. 1.34 x 10^5 M 1 point 54. How many moles of ammonia can be produced from 8.0 mole of hydrogen reacting with nitrogen? The balanced reaction is; 3 H2 + N2 -> 2 NH3 O A. 4.34 moles NH3 O B. 4.33 moles NH3 O C.5.33 moles NH3 2 points 56-57. A bottle of phosphoric acid is labeled "85.0% H3PO4 by mass; density = 1.689 g/cm3." Calculate the molarity of phosphoric acid in the solution A. 16.7 M O B. 12.2 M O C. 14.6 M O D. 8.67 M 3 points 58-60. What is the maximum volume of 0.25 M KCl(aq) that can be prepared from 75 g KCl(s)? * O A. 0.33L OB. 1.0L C. 4.0L O D.3.0L
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


