Question
5.18 The free expansion of a gas is a process where the total mean energy E remains constant. In connection with this process, the
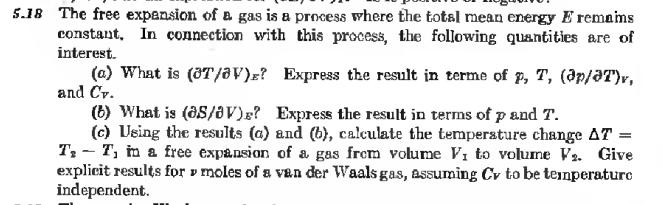
5.18 The free expansion of a gas is a process where the total mean energy E remains constant. In connection with this process, the following quantities are of interest. (a) What is (T/V)? Express the result in terme of p, T, (ap/aT)r, and Cv. (b) What is (as/V)? Express the result in terms of p and T. (c) Using the results (a) and (b), calculate the temperature change AT = T, T, in a free expansion of a gas frem volume V, to volume V2. Give explicit results for moles of a van der Waals gas, assuming Cv to be temperature independent.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
University Physics with Modern Physics
Authors: Hugh D. Young, Roger A. Freedman
14th edition
133969290, 321973615, 9780321973610, 978-0133977981
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



