Answered step by step
Verified Expert Solution
Question
1 Approved Answer
53. One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The
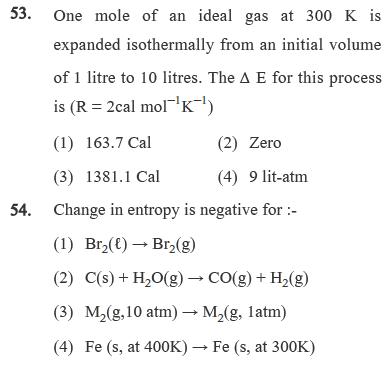
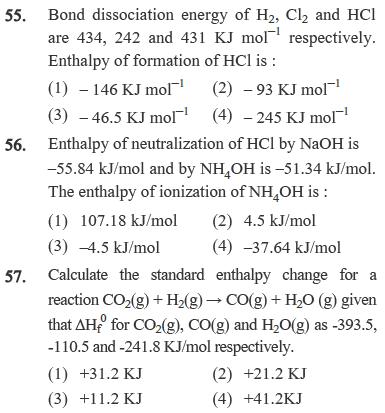
53. One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The A E for this process is (R = 2cal molK) (1) 163.7 Cal (2) Zero (3) 1381.1 Cal (4) 9 lit-atm 54. Change in entropy is negative for :- (1) Br2() Br2(g) (2) C(s) + H2O(g) CO(g) + H2(g) (3) M(g,10 atm) M(g, 1atm) (4) Fe (s, at 400K) Fe (s, at 300K) 55. Bond dissociation energy of H2, Cl and HCI are 434, 242 and 431 KJ mol respectively. Enthalpy of formation of HCl is: (1)-146 KJ mol (2) -93 KJ mol (3) -46.5 KJ mol (4) -245 KJ mol 56. Enthalpy of neutralization of HCl by NaOH is -55.84 kJ/mol and by NH4OH is -51.34 kJ/mol. The enthalpy of ionization of NH4OH is : (1) 107.18 kJ/mol (2) 4.5 kJ/mol (3) -4.5 kJ/mol (4) -37.64 kJ/mol 57. Calculate the standard enthalpy change for a reaction CO2(g) + H2(g) CO(g) + H2O (g) given that AH for CO2(g), CO(g) and H2O(g) as -393.5, -110.5 and -241.8 KJ/mol respectively. (1) +31.2 KJ (2) +21.2 KJ (3) +11.2 KJ (4) +41.2KJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1
55 The enthalpy of formation of HCl can be calculated using the bond dissociation energies of H2 Cl2 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


