Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A - Measuring pH Solution pH Calculated POH Calculated [H'] [OH] 1.0M Acetic Acid 2.38 0.00417 11.62 2.41 x 10-12 9.38 4.203 x
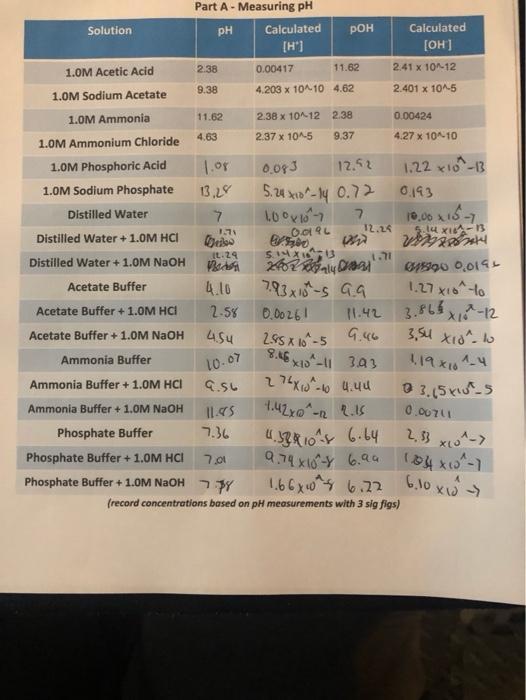

Part A - Measuring pH Solution pH Calculated POH Calculated [H'] [OH] 1.0M Acetic Acid 2.38 0.00417 11.62 2.41 x 10-12 9.38 4.203 x 1010 4.62 2.401 x 10^-5 1.0M Sodium Acetate 1.0M Ammonia 11.62 2.38 x 10-12 2.38 0.00424 4.63 2.37 x 10^-5 9.37 4.27 x 1010 1.0M Ammonium Chloride 1.0M Phosphoric Acid 1.or 0.093 12.52 1.22 x10-13 1.0M Sodium Phosphate 13,28 5.4 -1y 0.72 0193 10,00 x16-7 12.24 Distilled Water Distilled Water + 1.0M HCI IL.29 Distilled Water + 1.0M NAOH 1.27 xio-lo 3.FL Acetate Buffer 4.10 7.93x10-s G9 Acetate Buffer +1.0M HCI 2-58 0.00261 11.42 -12 Acetate Buffer + 1.0M NaOH 2.55 X 16 -5 G.46 Ammonia Buffer 10.07 x1-| 393 119X161-4 A 0 3.(5 x5 Ammonia Buffer + 1.0M HCI Ammonia Buffer + 1.0M NAOH 1.42xo^ 0.0071 -12 2, 3 xA-7 104x0-1 6,10 xI> Phosphate Buffer 36. 4.53R10 6.64 Phosphate Buffer + 1.0M HCI 7.01 9.74 X10Y 6.94 Phosphate Buffer + 1.0M NAOH 7Y 1,66x0 6.22 (record concentrations based on pH measurements with 3 sig figs) #5). Use your pH value to calculate the [NH'] of the 1.0M Ammonia. Show your work. #6). What is the % lonization of Ammonia? Show your work. #7). What is the calculated K, for Ammonia? Show your work.
Step by Step Solution
★★★★★
3.29 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


