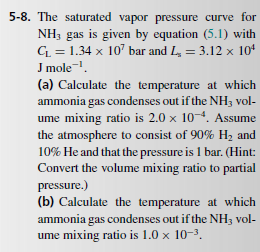

= 5-8. The saturated vapor pressure curve for NH; gas is given by equation (5.1) with Cu = 1.34 x 10 bar and L. = 3.12 x 104 J mole! (a) Calculate the temperature at which ammonia gas condenses out if the NH; vol- ume mixing ratio is 2.0 x 10-4. Assume the atmosphere to consist of 90% H, and 10% He and that the pressure is 1 bar. (Hint: Convert the volume mixing ratio to partial pressure.) (b) Calculate the temperature at which ammonia gas condenses out if the NH3 vol- ume mixing ratio is 1.0 x 10-3. 5.3 Clouds Earth's atmosphere contains a small (~1%) and highly variable amount of water vapor (Table E.12). The air is said to be saturated if the abun- dance of water vapor (or, in general, of any con- densable species under consideration) is at its max- imum vapor partial pressure. Under equilibrium conditions, air cannot contain more water vapor than indicated by its saturation vapor pressure curve. The saturation vapor pressure of H2O is sketched in Figure 5.4a, and given by the Clausius- Clapeyron equation of state: P=Ce-L/R) (5.1) where is the latent heat, Rgas the gas constant and a constant. = 5-8. The saturated vapor pressure curve for NH; gas is given by equation (5.1) with Cu = 1.34 x 10 bar and L. = 3.12 x 104 J mole! (a) Calculate the temperature at which ammonia gas condenses out if the NH; vol- ume mixing ratio is 2.0 x 10-4. Assume the atmosphere to consist of 90% H, and 10% He and that the pressure is 1 bar. (Hint: Convert the volume mixing ratio to partial pressure.) (b) Calculate the temperature at which ammonia gas condenses out if the NH3 vol- ume mixing ratio is 1.0 x 10-3. 5.3 Clouds Earth's atmosphere contains a small (~1%) and highly variable amount of water vapor (Table E.12). The air is said to be saturated if the abun- dance of water vapor (or, in general, of any con- densable species under consideration) is at its max- imum vapor partial pressure. Under equilibrium conditions, air cannot contain more water vapor than indicated by its saturation vapor pressure curve. The saturation vapor pressure of H2O is sketched in Figure 5.4a, and given by the Clausius- Clapeyron equation of state: P=Ce-L/R) (5.1) where is the latent heat, Rgas the gas constant and a constant








