Answered step by step
Verified Expert Solution
Question
1 Approved Answer
.59 The structure of montelukast, an antiasthma drug, is shown here. (a) Use Table L.8 to identify the most acidic and most basic sites in
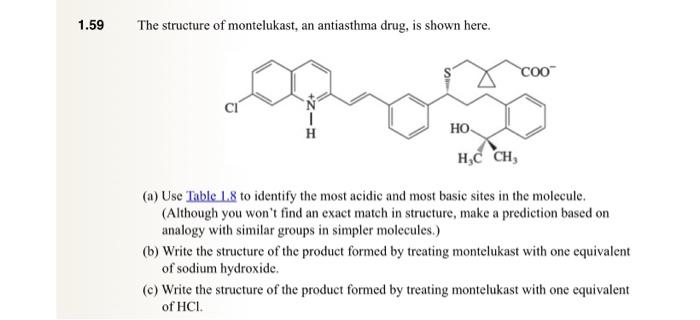
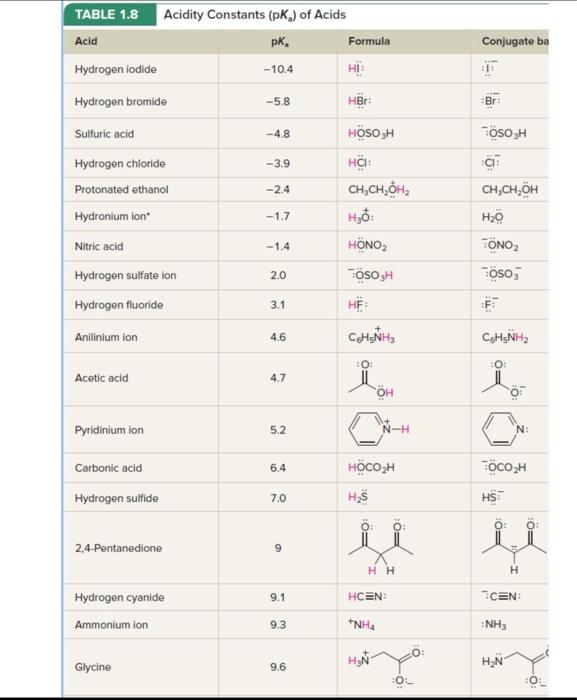
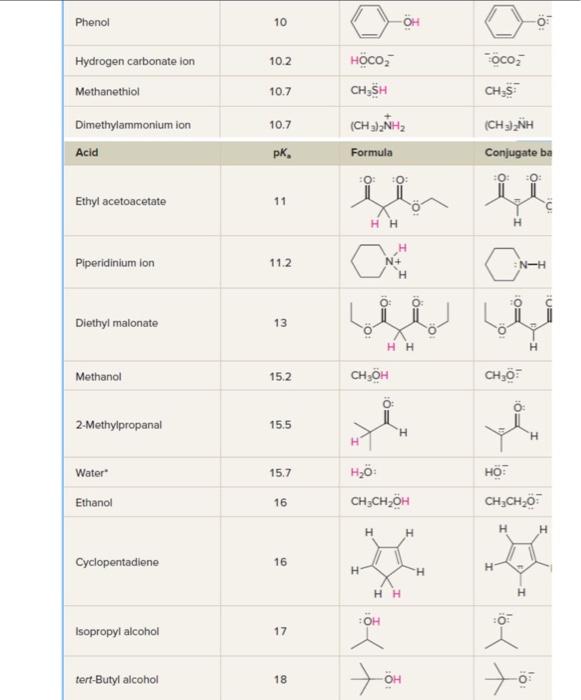
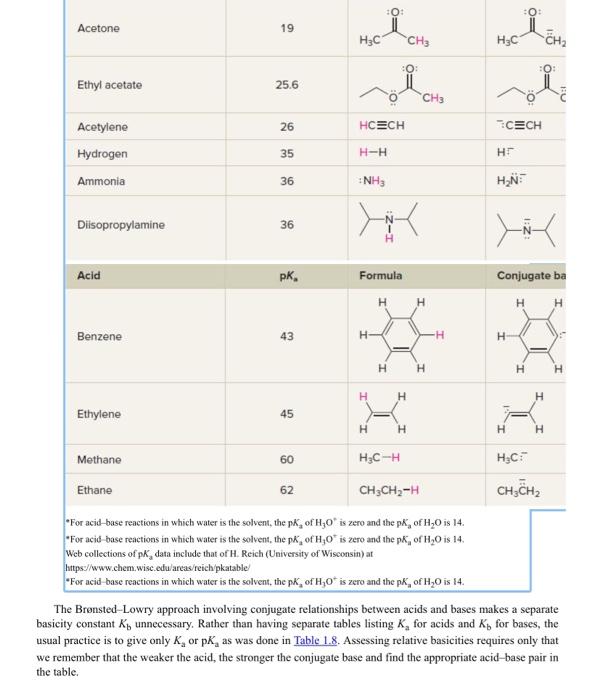 .59 The structure of montelukast, an antiasthma drug, is shown here. (a) Use Table L.8 to identify the most acidic and most basic sites in the molecule. (Although you won't find an exact match in structure, make a prediction based on analogy with similar groups in simpler molecules.) (b) Write the structure of the product formed by treating montelukast with one equivalent of sodium hydroxide. (c) Write the structure of the product formed by treating montelukast with one equivalent of HCl. TABLE 1.8 Acidity Constants (pKa) of Acids -For acid base reactions in which water is the solvent, the pKa of H3O+is zero and the pKa of H2O is 14. Web collections of pKa data include that of H. Reich (University of Wisconsin) at hitpsi//www.chem.wisc.edu/areas/reichpkatable/ "For acid base reactions in which water is the solvent, the pKa of H3O+is zero and the pKa of H2O is 4. The Bronsted-Lowry approach involving conjugate relationships between acids and bases makes a separate basicity constant Kb unnecessary. Rather than having separate tables listing Ka for acids and Kb for bases, the usual practice is to give only Ka or pKa as was done in Table 1.8. Assessing relative basicities requires only that we remember that the weaker the acid, the stronger the conjugate base and find the appropriate acid-base pair in the table
.59 The structure of montelukast, an antiasthma drug, is shown here. (a) Use Table L.8 to identify the most acidic and most basic sites in the molecule. (Although you won't find an exact match in structure, make a prediction based on analogy with similar groups in simpler molecules.) (b) Write the structure of the product formed by treating montelukast with one equivalent of sodium hydroxide. (c) Write the structure of the product formed by treating montelukast with one equivalent of HCl. TABLE 1.8 Acidity Constants (pKa) of Acids -For acid base reactions in which water is the solvent, the pKa of H3O+is zero and the pKa of H2O is 14. Web collections of pKa data include that of H. Reich (University of Wisconsin) at hitpsi//www.chem.wisc.edu/areas/reichpkatable/ "For acid base reactions in which water is the solvent, the pKa of H3O+is zero and the pKa of H2O is 4. The Bronsted-Lowry approach involving conjugate relationships between acids and bases makes a separate basicity constant Kb unnecessary. Rather than having separate tables listing Ka for acids and Kb for bases, the usual practice is to give only Ka or pKa as was done in Table 1.8. Assessing relative basicities requires only that we remember that the weaker the acid, the stronger the conjugate base and find the appropriate acid-base pair in the table




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


