Answered step by step
Verified Expert Solution
Question
1 Approved Answer
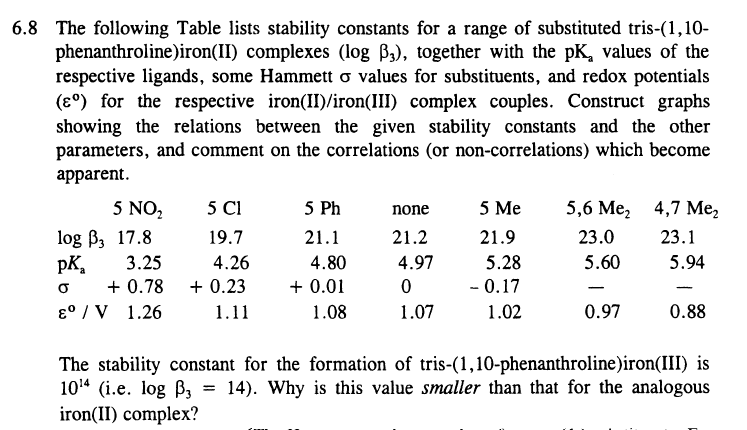
6 . 8 The following Table lists stability constants for a range of substituted tris - ( 1 , 1 0 - phenanthroline ) iron
The following Table lists stability constants for a range of substituted tris
phenanthrolineironII complexes together with the values of the
respective ligands, some Hammett values for substituents, and redox potentials
for the respective ironIIironIII complex couples. Construct graphs
showing the relations between the given stability constants and the other
parameters, and comment on the correlations or noncorrelations which become
apparent.
The stability constant for the formation of trisphenanthrolineironIII is
ie Why is this value smaller than that for the analogous
ironII complex?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


