Question
6. a) Consider the reaction N2(8) + 3H2(g) 2NH3(g) AHxn -92.6 kJ If 2 moles of N2 reacts with 6 moles of H2 to
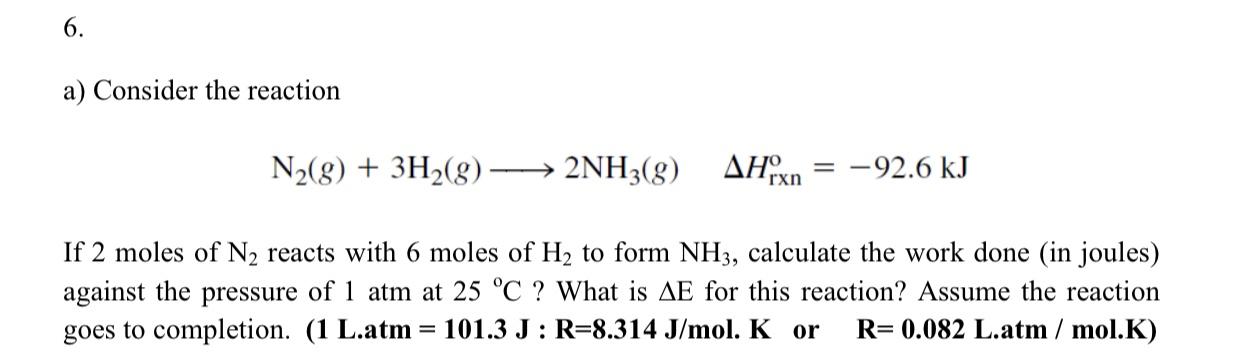
6. a) Consider the reaction N2(8) + 3H2(g) 2NH3(g) AHxn -92.6 kJ If 2 moles of N2 reacts with 6 moles of H2 to form NH3, calculate the work done (in joules) against the pressure of 1 atm at 25 C ? What is AE for this reaction? Assume the reaction goes to completion. (1 L.atm = 101.3 J : R=8.314 J/mol. K or R= 0.082 L.atm / mol.K)
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



