Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6. At 500C and 93.2 kPa, the mass density of sulfur vapour is 3.710 kg m-3. What is the molecular formula of sulfur under



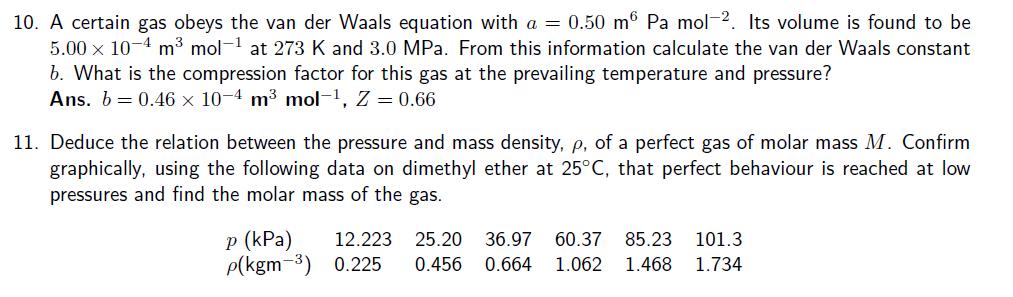

6. At 500C and 93.2 kPa, the mass density of sulfur vapour is 3.710 kg m-3. What is the molecular formula of sulfur under these conditions? 7. Calculate the mass of water vapour present in a room of volume 400 m that contains air at 27C on a day when the relative humidity is 60 per cent. Given saturated vapour density at 27C is 25.7 g.m-3 8. Given that the density of air at 740 Torr and 27C is 1.146 kg m calculate the mole fraction and partial pressure of nitrogen and oxygen assuming that (a) air consists only of these two gases, (b) air also contains 1.0 mole per cent Ar. 10. A certain gas obeys the van der Waals equation with a = 0.50 m Pa mol-2. Its volume is found to be 5.00 x 10-4 m mol-1 at 273 K and 3.0 MPa. From this information calculate the van der Waals constant b. What is the compression factor for this gas at the prevailing temperature and pressure? Ans. 60.46 10-4 m mol-1, Z = 0.66 11. Deduce the relation between the pressure and mass density, p, of a perfect gas of molar mass M. Confirm graphically, using the following data on dimethyl ether at 25C, that perfect behaviour is reached at low pressures and find the molar mass of the gas. p (kPa) 12.223 25.20 36.97 60.37 85.23 p(kgm-3) 0.225 0.456 0.664 1.062 1.468 101.3 1.734 9. The densities of air at -85C, 0C, and 100C are 1.877 g dm, 1.294 g dm, and 0.946 g dm, respectively. From these data, and assuming that air obeys Charles's law, determine a value for the absolute zero of temperature in degrees Celsius.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


