6 ch 1 12 4 15 Calculate the molar mass of each element or compound below. Use the periodic table provided above. 1. The
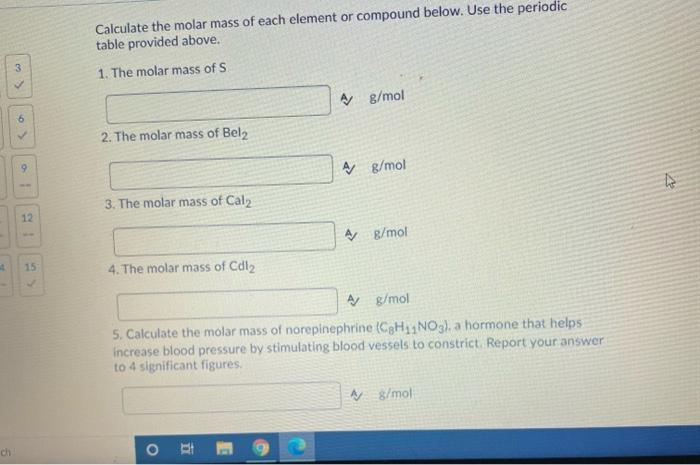
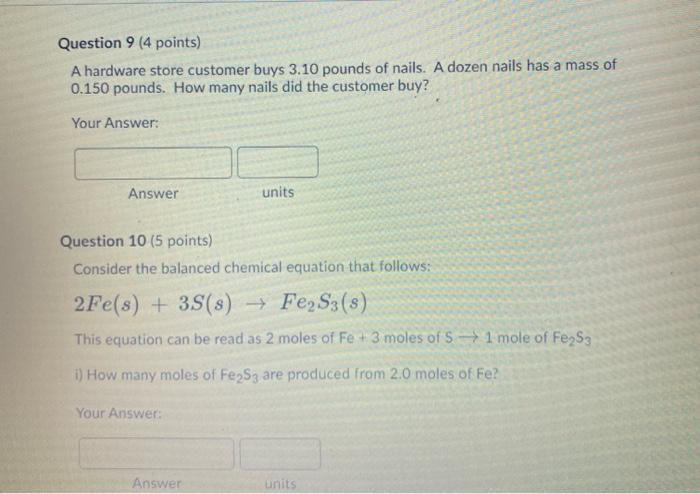



6 ch 1 12 4 15 Calculate the molar mass of each element or compound below. Use the periodic table provided above. 1. The molar mass of S 2. The molar mass of Bel2 3. The molar mass of Cal2 4. The molar mass of Cdl O A g/mol HI A g/mol A g/mol 5. Calculate the molar mass of norepinephrine (C8H1NO3), a hormone that helps increase blood pressure by stimulating blood vessels to constrict. Report your answer to 4 significant figures. A g/mol A g/mol V 1: 3 9 11 12 14 15 Question 8 (5 points) Look at the units of molar mass and describe what kind of conversion factor it is and what it helps you do? f Y Paragraph V BIU *** 9 Fax / Question 9 (4 points) A hardware store customer buys 3.10 pounds of nails. A dozen nails has a mass of 0.150 pounds. How many nails did the customer buy? Your Answer: Answer Question 10 (5 points) Consider the balanced chemical equation that follows: 2Fe(s) + 3S(s) FeS3(s) This equation can be read as 2 moles of Fe + 3 moles of S1 mole of FeS3 i) How many moles of Fe2S3 are produced from 2.0 moles of Fe? Your Answer: units Answer units 3 6 19 1 12 15 Question 11 (5 points) Consider the balanced chemical equation that follows: 2Fe(s) + 3S(s) FeS3(s) This equation can be read as 2 moles of Fe + 3 moles of S 1 mole of Fe2S3 ii) How many moles of Fe2S3 are produced from 29.0 moles of S? Your Answer: Answer units 2 3 55 6 8 9 11 12 14 15 Question 12 (5 points) Consider the balanced chemical equation that follows: 2Fe(s) + 3S(s) Fe2S3 (8) This equation can be read as 2 moles of Fe + 3 moles of 5 1 mole of Fe2S3 iii) How many moles of S react with 7.0 moles of Fe? Your Answer: Answer units 5 8 11 6> 14 9. 12 15 Question 14 (4 points) Iron (III) oxide reacts with carbon to give iron and carbon monoxide: Fe2O3(s) + 3C(s)2Fe(s) + 3CO(g) How many grams of C are required to react with 32.7 g of FeO3 Your Answer: Answer units
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
14 Consider Fe s 3 CC 2Fes 3co g dow m...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


