Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6. In a PEM fuel cell with an active area of 300cm2 and a net output voltage of 0.9V, where hydrogen and air are used
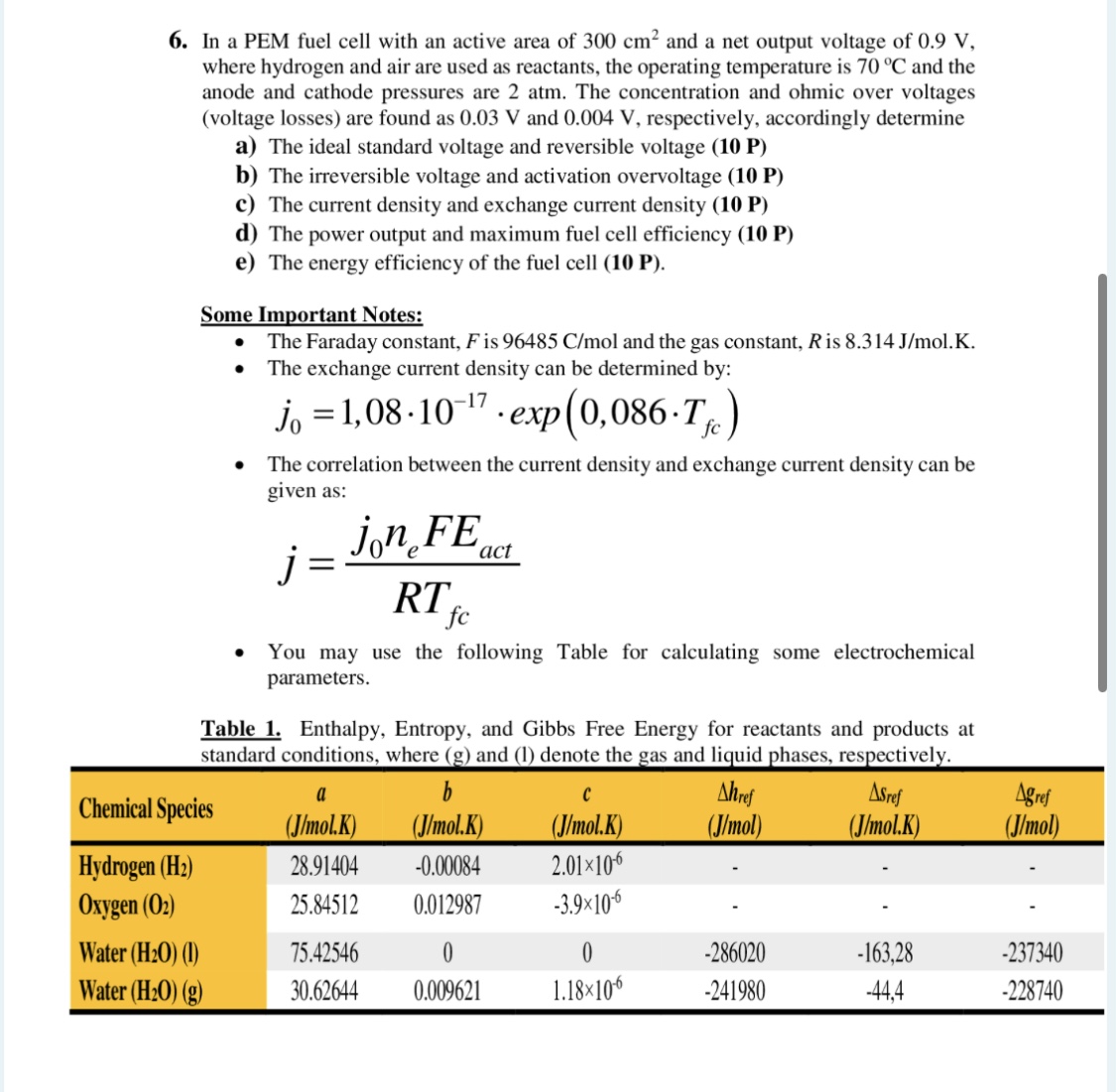 6. In a PEM fuel cell with an active area of 300cm2 and a net output voltage of 0.9V, where hydrogen and air are used as reactants, the operating temperature is 70C and the anode and cathode pressures are 2atm. The concentration and ohmic over voltages (voltage losses) are found as 0.03V and 0.004V, respectively, accordingly determine a) The ideal standard voltage and reversible voltage (10 P) b) The irreversible voltage and activation overvoltage (10 P) c) The current density and exchange current density (10 P) d) The power output and maximum fuel cell efficiency (10 P) e) The energy efficiency of the fuel cell (10 P). Some Important Notes: - The Faraday constant, F is 96485C/mol and the gas constant, R is 8.314J/mol.K. - The exchange current density can be determined by: j0=1,081017exp(0,086Tfc) - The correlation between the current density and exchange current density can be given as: n=Rfcj0neFact - You may use the following Table for calculating some electrochemical parameters. Table 1. Enthalpy, Entropy, and Gibbs Free Energy for reactants and products at
6. In a PEM fuel cell with an active area of 300cm2 and a net output voltage of 0.9V, where hydrogen and air are used as reactants, the operating temperature is 70C and the anode and cathode pressures are 2atm. The concentration and ohmic over voltages (voltage losses) are found as 0.03V and 0.004V, respectively, accordingly determine a) The ideal standard voltage and reversible voltage (10 P) b) The irreversible voltage and activation overvoltage (10 P) c) The current density and exchange current density (10 P) d) The power output and maximum fuel cell efficiency (10 P) e) The energy efficiency of the fuel cell (10 P). Some Important Notes: - The Faraday constant, F is 96485C/mol and the gas constant, R is 8.314J/mol.K. - The exchange current density can be determined by: j0=1,081017exp(0,086Tfc) - The correlation between the current density and exchange current density can be given as: n=Rfcj0neFact - You may use the following Table for calculating some electrochemical parameters. Table 1. Enthalpy, Entropy, and Gibbs Free Energy for reactants and products at Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


