Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(6%) Problem 9: Soda from a mg = 12 oz can at temperature Ts = 11C is poured in its entirety into a glass
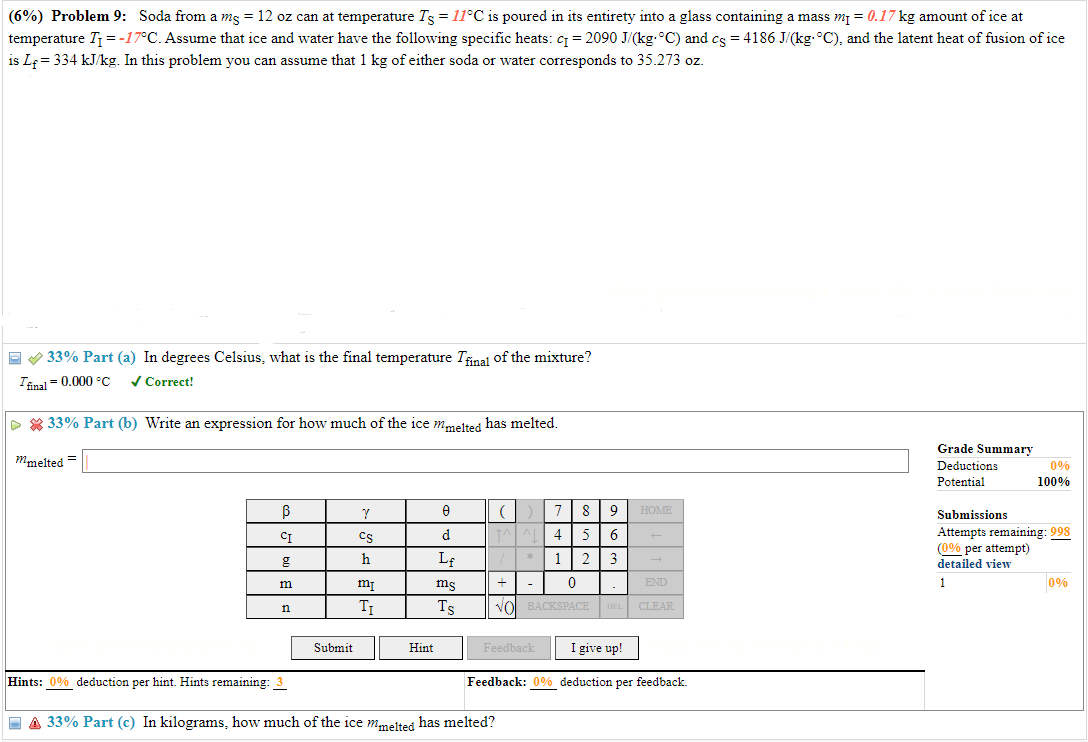
(6%) Problem 9: Soda from a mg = 12 oz can at temperature Ts = 11C is poured in its entirety into a glass containing a mass m = 0.17 kg amount of ice at temperature T = -17C. Assume that ice and water have the following specific heats: c = 2090 J/(kg. C) and cs = 4186 J/(kg. C), and the latent heat of fusion of ice is Lf=334 kJ/kg. In this problem you can assume that 1 kg of either soda or water corresponds to 35.273 oz. Correct! >33% Part (a) In degrees Celsius, what is the final temperature Tfinal of the mixture? Ifinal=0.000 C 33% Part (b) Write an expression for how much of the ice mmelted has melted. mmelted = Grade Summary Deductions Potential Submissions 0% 100% Attempts remaining: 998 (0% per attempt) detailed view 0 V () 7 8 9 HOME CI CS d 1^4 5 6 g h Lf * 1 2 3 + m mi ms 0 END 1 n TI Ts VO BACKSPACE DEL CLEAR Hint Feedback I give up! Submit Hints: 0% deduction per hint. Hints remaining: 3 Feedback: 0% deduction per feedback. A 33% Part (c) In kilograms, how much of the ice mmelted has melted? 0%
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the pressure at the top gauge we can apply Bernoullis equation between points A and B where ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
663d92b02696d_965137.pdf
180 KBs PDF File
663d92b02696d_965137.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


