Question
6. The water-gas shift reaction (WGSR) is an important industrial reaction that is used in the manufacture of ammonia. The WGSR is the reaction
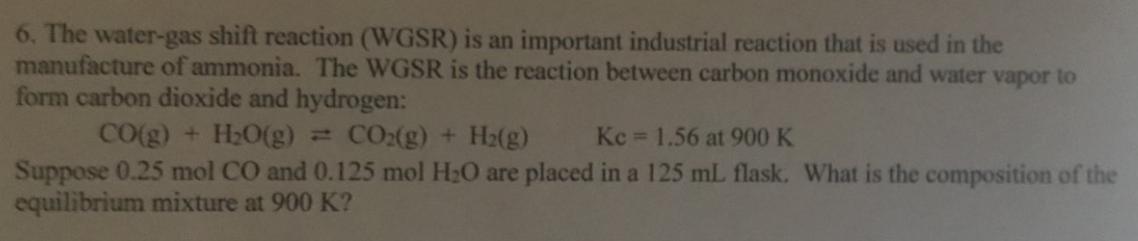
6. The water-gas shift reaction (WGSR) is an important industrial reaction that is used in the manufacture of ammonia. The WGSR is the reaction between carbon monoxide and water vapor to form carbon dioxide and hydrogen: CO(g) + H20(g) = CO:(g) + H2(g) Kc 1.56 at 900 K Suppose 0.25 mol CO and 0.125 mol H20 are placed in a 125 mL flask. What is the composition of the equilibrium mixture at 900 K?
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
colgo Hzg MzO Equilibrium content ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



