Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6. Using the data on the chart, calculate the average atomic mass for element sulfur. 7. A sample of H2SO4 gas is founded to have
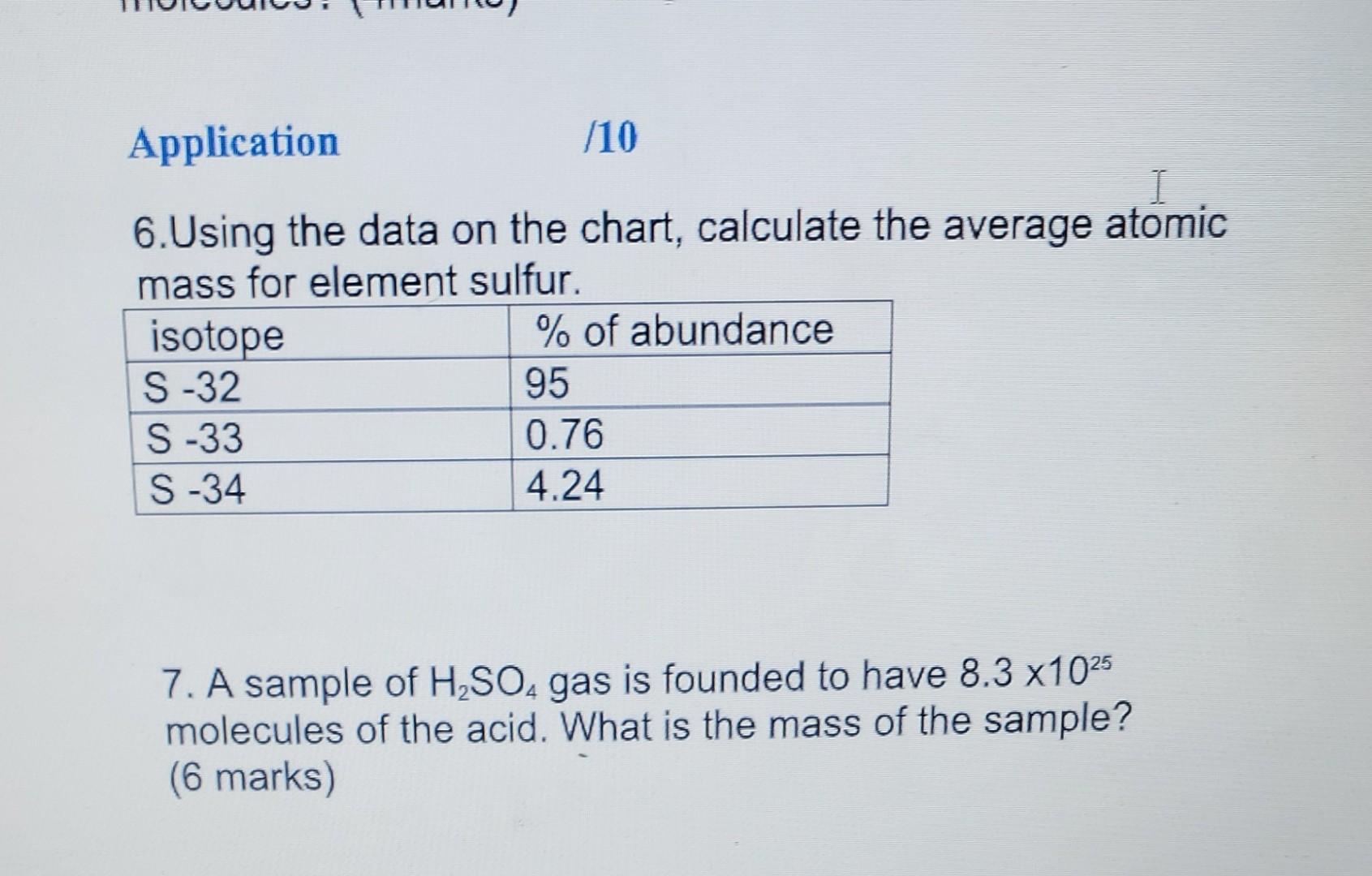
6. Using the data on the chart, calculate the average atomic mass for element sulfur. 7. A sample of H2SO4 gas is founded to have 8.31025 molecules of the acid. What is the mass of the sample? (6 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


