Answered step by step
Verified Expert Solution
Question
1 Approved Answer
61. The solubility product of CuS, AgS and HgS are 10 37, 104 and 1054 respectively. The solubility of these sulphides will be in
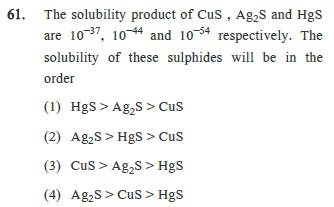
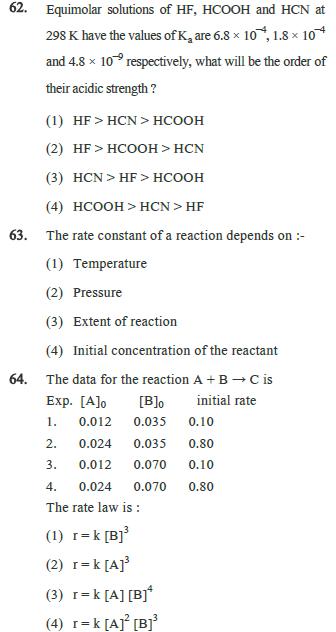
61. The solubility product of CuS, AgS and HgS are 10 37, 104 and 1054 respectively. The solubility of these sulphides will be in the order (1) HgS> AgS > Cus (2) AgS>HgS > CuS (3) CuS AgS > HgS (4) AgS>CuS > HgS 62. Equimolar solutions of HF, HCOOH and HCN at 298 K. have the values of K, are 6.8 10, 1.8 104 and 4.8 10 respectively, what will be the order of their acidic strength? (1) HF>HCN>HCOOH (2) HFHCOOH > HCN. (3) HCN>HF> HCOOH (4) HCOOH>HCN>HF 63. The rate constant of a reaction depends on :- (1) Temperature (2) Pressure (3) Extent of reaction (4) Initial concentration of the reactant 64. The data for the reaction A + B C is Exp. [A]o [B]0 initial rate 1. 0.012 0.035 0.10 2. 0.024 0.035 0.80 3. 0.012 0.070 0.10 4. 0.024 0.070 0.80 The rate law is: (1) r = k [B] (2) r=k[A] (3) r=k [A] [B] (4) r= k [A] [B]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
61 The correct answer is 3 CuS Ag2S HgS Explanation The solubility product Ksp values of the given s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


