Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6.11. Zeldovich Equation for NO Synthesis in Quasi-Equilibrium Thermal Systems. Using the Zeldovich equation (6-62), calculate the time required for reaching quasi- equilibrium concentration of

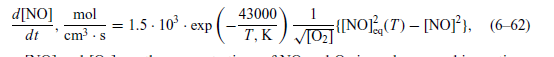
6.11. Zeldovich Equation for NO Synthesis in Quasi-Equilibrium Thermal Systems. Using the Zeldovich equation (6-62), calculate the time required for reaching quasi- equilibrium concentration of nitrogen oxides (NOx) in stoichiometric N2-02 atmospheric- pressure mixture at different thermal plasma temperatures: 3000, 3500, and 4000 K. Analyz- ing activation energies in the Zeldovich equation, find out the limiting reaction responsible for establishing of the quasi-equilibrium concentration of NOx| mol 1 d[NO] dt 1.5. 103. exp cm.s ( 43000 T, K 2) VOZ INOL_(T) [NO]), (6-62)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


