Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7. [25 points] A subsurface site has spilled a nonaqueous phase liquid that is composed of only two compounds, each at a mole fraction
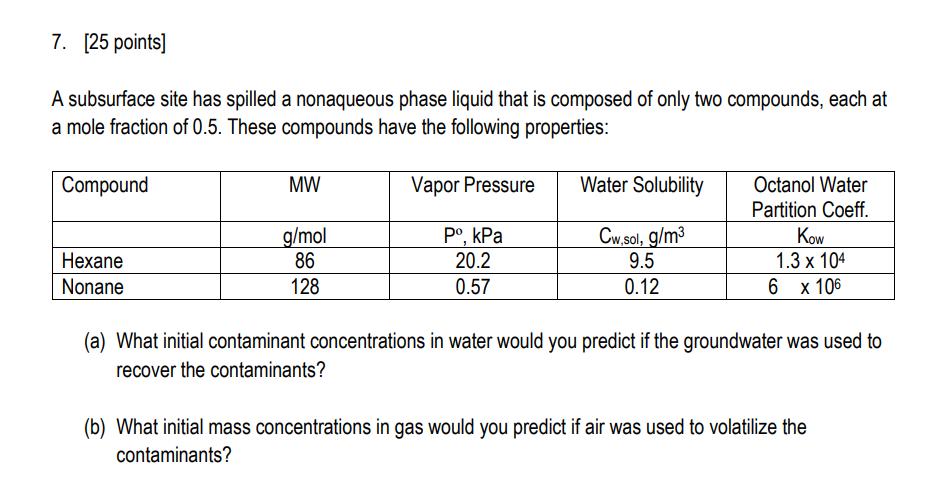


7. [25 points] A subsurface site has spilled a nonaqueous phase liquid that is composed of only two compounds, each at a mole fraction of 0.5. These compounds have the following properties: Compound Hexane Nonane MW Vapor Pressure Water Solubility Octanol Water Partition Coeff. g/mol PokPa Cw.sol, g/m 86 20.2 9.5 Kow 1.3 x 104 128 0.57 0.12 6 x 106 (a) What initial contaminant concentrations in water would you predict if the groundwater was used to recover the contaminants? (b) What initial mass concentrations in gas would you predict if air was used to volatilize the contaminants? 8. [25 points] An experiment to determine compound partitioning among air, water, and solid phases was conducted by adding 1 gram of the compound to a reactor that had 1 m of air, 10-3 m of water, and 100 g of solid. The reactor was at atmospheric pressure, and at a constant temperature of 20C (293K). The compound has a molecular weight of 110 g mole-1. Once equilibrium is reached, the air has a mass concentration of 0.400 g m-3, and the water has a mass concentration of 250 g m-. a. Determine the value of the Henry law constant for the compound, HCA. b. Given that adsorption between water and the solid phase for this compound is linear, determine the isotherm constant. c. If the solid phase were completely removed from the air and water, and then resuspended in 10-3 m of initially clean water, what would be the contaminant concentration in the water once equilibrium is attained?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


