Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7 A compound shows a molecular ion peak in its mass spectrum at 58 m/z, an IHD of 1 and a sharp absorption at about
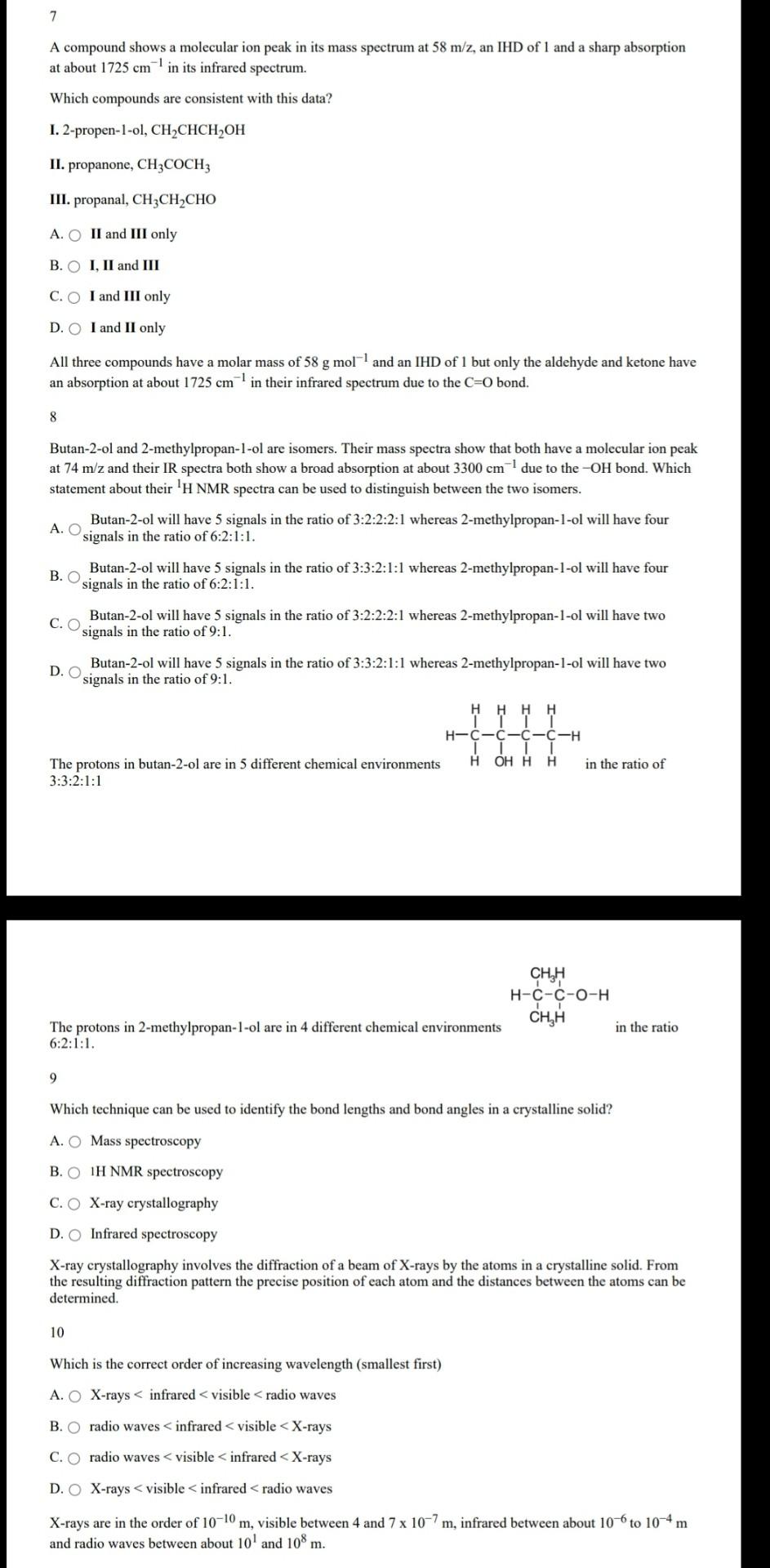
7 A compound shows a molecular ion peak in its mass spectrum at 58 m/z, an IHD of 1 and a sharp absorption at about 1725 cm in its infrared spectrum. Which compounds are consistent with this data? 1. 2-propen-1-ol, CHCHCH OH II. propanone, CH3COCH3 III. propanal, CH2CH2CHO A. O II and III only B. O I, II and III C. O I and III only D. O I and II only All three compounds have a molar mass of 58 g moll and an IHD of 1 but only the aldehyde and ketone have an absorption at about 1725 cm in their infrared spectrum due to the C=0 bond. 8 Butan-2-ol and 2-methylpropan-1-ol are isomers. Their mass spectra show that both have a molecular ion peak at 74 m/z and their IR spectra both show a broad absorption at about 3300 cm due to the -OH bond. Which statement about their 'H NMR spectra can be used to distinguish between the two isomers. Butan-2-ol will have 5 signals in the ratio of 3:2:2:2:1 whereas 2-methylpropan-1-ol will have four A. O signals in the ratio of 6:2:1:1. B. O Butan-2-ol will have 5 signals in the ratio of 3:3:2:1:1 whereas 2-methylpropan-1-ol will have four signals in the ratio of 6:2:1:1. Butan-2-ol will have 5 signals in the ratio of 3:2:2:2:1 whereas 2-methylpropan-1-ol will have two C. O signals in the ratio of 9:1. Butan-2-ol will have 5 signals in the ratio of 3:3:2:1:1 whereas 2-methylpropan-1-ol will have two D. O signals in the ratio of 9:1. H H-C-C-C-C-H TL H OHHH The protons in butan-2-ol are in 5 different chemical environments in the ratio of 3:3:2:1:1 CHA H-C-C-0-H CH,H The protons in 2-methylpropan-1-ol are in 4 different chemical environments 6:2:1:1. in the ratio 9 Which technique can be used to identify the bond lengths and bond angles in a crystalline solid? A. O Mass spectroscopy B. O IH NMR spectroscopy C. O X-ray crystallography D. O Infrared spectroscopy X-ray crystallography involves the diffraction of a beam of X-rays by the atoms in a crystalline solid. From the resulting diffraction pattern the precise position of each atom and the distances between the atoms can be determined. 10 Which is the correct order of increasing wavelength (smallest first) A. O X-rays
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


