Question
7. Consider the following chain for dry CO oxidation: k CO + O CO + O K 0 + 0 + M 03 +
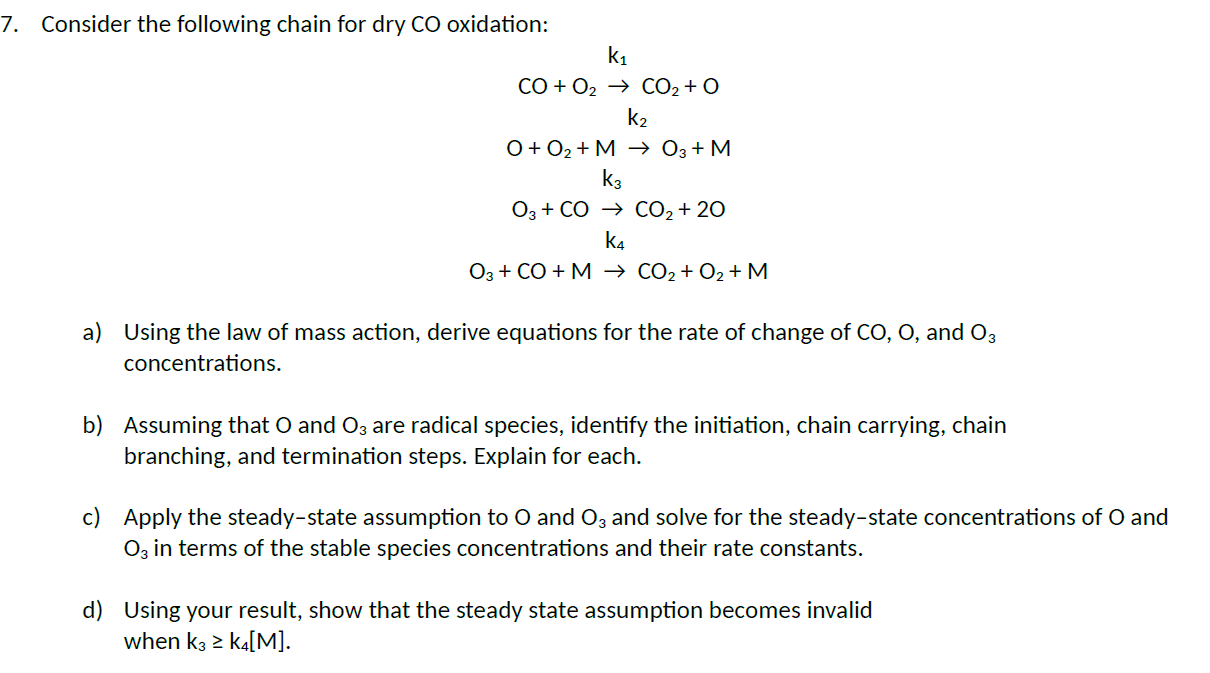
7. Consider the following chain for dry CO oxidation: k CO + O CO + O K 0 + 0 + M 03 + M K3 O3 + CO CO + 20 K4 O3 + CO + M CO + O + M a) Using the law of mass action, derive equations for the rate of change of CO, O, and 03 concentrations. b) Assuming that O and O3 are radical species, identify the initiation, chain carrying, chain branching, and termination steps. Explain for each. c) Apply the steady-state assumption to O and O3 and solve for the steady-state concentrations of O and O3 in terms of the stable species concentrations and their rate constants. d) Using your result, show that the steady state assumption becomes invalid when k3 > K4[M].
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Peter Atkins
7th Edition
978-0716735397, 716735393, 978-0716743880
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



