Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7. Cu(s) + Sn+(0.001M) Cu+ (0.01M) + Sn(s) The Gibbs free energy change for the above reaction at 298 K is x x 10
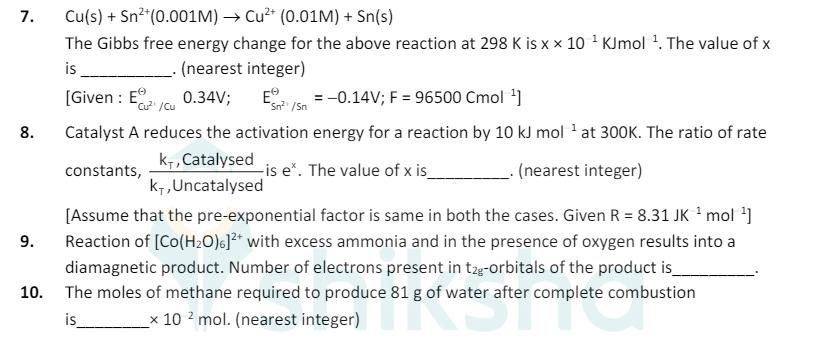
7. Cu(s) + Sn+(0.001M) Cu+ (0.01M) + Sn(s) The Gibbs free energy change for the above reaction at 298 K is x x 10 KJmol 1. The value of x _. (nearest integer) is [Given: E Cu/Cu 0.34V; E =-0.14V; F = 96500 Cmol ] 8. 1 9. 10. Sn/Sn Catalyst A reduces the activation energy for a reaction by 10 kJ mol at 300K. The ratio of rate K+, Catalysed K+, Uncatalysed constants, is ex. The value of x is_ . (nearest integer) [Assume that the pre-exponential factor is same in both the cases. Given R = 8.31 JK mol ] Reaction of [Co(H2O)6]+ with excess ammonia and in the presence of oxygen results into a diamagnetic product. Number of electrons present in t2g-orbitals of the product is_ The moles of methane required to produce 81 g of water after complete combustion is x 102 mol. (nearest integer)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


