Question
7. How many atoms are present in 10 grams of Sodium (Na) (2.0 points) (Avogadro's number is: 6.022 X 102) 8. How many grams
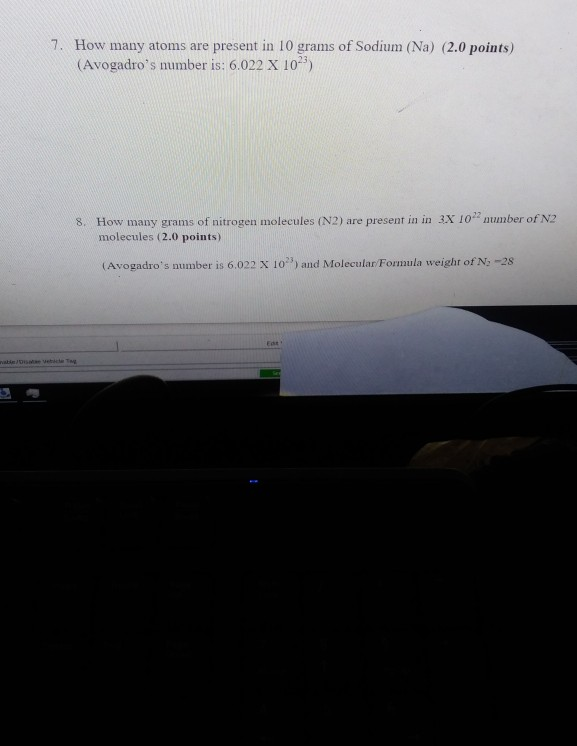
7. How many atoms are present in 10 grams of Sodium (Na) (2.0 points) (Avogadro's number is: 6.022 X 102) 8. How many grams of nitrogen molecules (N2) are present in in 3X 1022 number of N2 molecules (2.0 points) (Avogadro's number is 6.022 X 10") and Molecular/Formula weight of N-28 vable/Disabe vehicle Tag 1 Eats
Step by Step Solution
3.55 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
23 7 Na Ansuers Mass of sodium log Alass of sodium atom23g 6032x103 atoms 23g l...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



