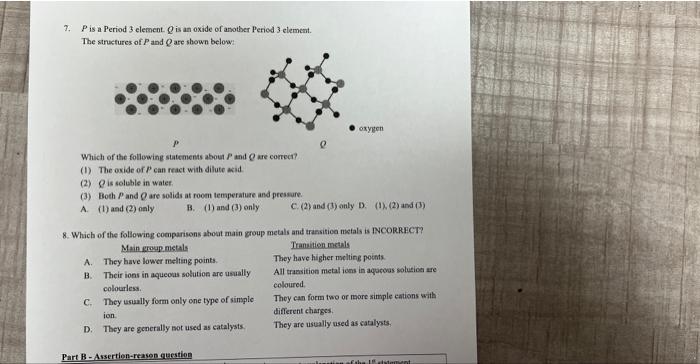
7. P is a Period 3 element Q is an oxide of another Peried 3 elemeat. The structures of P and Q are shown below: Which of the follewing statements about P and Q we correct? (1) The oxide of p can react with dilute akid. (2) Q is soluble in water. (3) Both P and Q are sotids at room temperature and pressure. A. (1) and (2) oaly B. (1) and (3) enly C. (2) and (3) only D. (1), (2) and (3) 8. Which of the following comparisons aboat main group metals and transitica metals is NNCORRACT? Main group metals Transition inctals A. They have lower melting points. They have higher melting points. B. Their ions in aqueoas solution are usually All trassition metal ions in aqacoss solutice are colourless. coloured. C. They uswally form only one type of simple They ean form two or more simple eations with: ion. different charges. D. They are generally not used as catalysts. They are usually used as catalysts, D. They are generally not used as catalysts. different charges. They are usually used as catalysts. Part B - Assertion-reason question A Both statements are true and the 2ed statement is a correct explanation of the 1tt statement. B Both statements are true but the 2ad statement is NOT a correct explabation of the 1" statement. The 111 statement is false but the 2rd statement is true. Both statements are false. End of Paper ** 1. Which of the following elements has the lowest melting point? A. Sulphar B. Chlorine C. Sodium D. Pbosphorus 2. Which of the following statements about the physical properties of Period 2 elements is INCORRECT? A. There is a gradual change from metals, semi-metals to non-metals. B. Neon has the lowest melting point across the period. C. Carbon has the highest melting point across the period. D. Going from Group I to Group III, the electrical conductivities of elements at room temperature increase. 3. The melting point of sulphur is higher than that of white phosphorus because (1) sulphur has a larger atomic number. (2) sulpbur molecules are larger than white phosphorus molecules. (3) S-S bonds are stronger than P-P bonds. A. (1) only B. (2) oaly C. (1) and (3) only D. (2) and (3) only 4. Which of the following oxides can react with dilute alkalis? (1) Al2O3 (2) SiO2 (3) SO2 A. (I) and ( 2 ) only B. (1) and ( 3 ) only. C. (2) and (3) only D. (1), (2) and (3) 5. Which of the following statements aboat the melting points of carbon and silicon is correet? A. Carbon has a bigher melting point than silicon because carbon has a giant covalent structure but silicon does not. B. Carbon has a higher melting point than silicon because the covalent bonds in carbon are stronger than those in silicon. A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 5. Which of the following statements about the melting points of carbon and silicon is correct? A. Carbon has a higher melting point than silicon because carbon has a giant covalent structure but silicon does not. B. Carbon has a higher melting point than silicon because the covalent bonds in carbon are stronger than those in silicon. C. Silicon has a higher melting point than carbon because it has a greater relative atomic mass and hence stronger van der Waals' forces between molecules. D. Silicon has a higher melting point than carbon because it has a greater relative atomic mass and hence stronger covalent bonds between atoms. 6. Which of the following statements about the non-metal oxides of Period 3 are correet? (1) They are covalent oxides. (2) They have simple molecular structures. (3) They react with dilute alkalis to form salts and water. A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 7. P is a Period 3 element Q is an oxide of another Peried 3 elemeat. The structures of P and Q are shown below: Which of the follewing statements about P and Q we correct? (1) The oxide of p can react with dilute akid. (2) Q is soluble in water. (3) Both P and Q are sotids at room temperature and pressure. A. (1) and (2) oaly B. (1) and (3) enly C. (2) and (3) only D. (1), (2) and (3) 8. Which of the following comparisons aboat main group metals and transitica metals is NNCORRACT? Main group metals Transition inctals A. They have lower melting points. They have higher melting points. B. Their ions in aqueoas solution are usually All trassition metal ions in aqacoss solutice are colourless. coloured. C. They uswally form only one type of simple They ean form two or more simple eations with: ion. different charges. D. They are generally not used as catalysts. They are usually used as catalysts, D. They are generally not used as catalysts. different charges. They are usually used as catalysts. Part B - Assertion-reason question A Both statements are true and the 2ed statement is a correct explanation of the 1tt statement. B Both statements are true but the 2ad statement is NOT a correct explabation of the 1" statement. The 111 statement is false but the 2rd statement is true. Both statements are false. End of Paper ** 1. Which of the following elements has the lowest melting point? A. Sulphar B. Chlorine C. Sodium D. Pbosphorus 2. Which of the following statements about the physical properties of Period 2 elements is INCORRECT? A. There is a gradual change from metals, semi-metals to non-metals. B. Neon has the lowest melting point across the period. C. Carbon has the highest melting point across the period. D. Going from Group I to Group III, the electrical conductivities of elements at room temperature increase. 3. The melting point of sulphur is higher than that of white phosphorus because (1) sulphur has a larger atomic number. (2) sulpbur molecules are larger than white phosphorus molecules. (3) S-S bonds are stronger than P-P bonds. A. (1) only B. (2) oaly C. (1) and (3) only D. (2) and (3) only 4. Which of the following oxides can react with dilute alkalis? (1) Al2O3 (2) SiO2 (3) SO2 A. (I) and ( 2 ) only B. (1) and ( 3 ) only. C. (2) and (3) only D. (1), (2) and (3) 5. Which of the following statements aboat the melting points of carbon and silicon is correet? A. Carbon has a bigher melting point than silicon because carbon has a giant covalent structure but silicon does not. B. Carbon has a higher melting point than silicon because the covalent bonds in carbon are stronger than those in silicon. A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 5. Which of the following statements about the melting points of carbon and silicon is correct? A. Carbon has a higher melting point than silicon because carbon has a giant covalent structure but silicon does not. B. Carbon has a higher melting point than silicon because the covalent bonds in carbon are stronger than those in silicon. C. Silicon has a higher melting point than carbon because it has a greater relative atomic mass and hence stronger van der Waals' forces between molecules. D. Silicon has a higher melting point than carbon because it has a greater relative atomic mass and hence stronger covalent bonds between atoms. 6. Which of the following statements about the non-metal oxides of Period 3 are correet? (1) They are covalent oxides. (2) They have simple molecular structures. (3) They react with dilute alkalis to form salts and water. A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3)