Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(7 pts) WRITTEN: ENTER ANSWER ON WRITTEN TEMPLATE Consider the molecule PSBr. P is the central atom in the molecule, and the 5 and

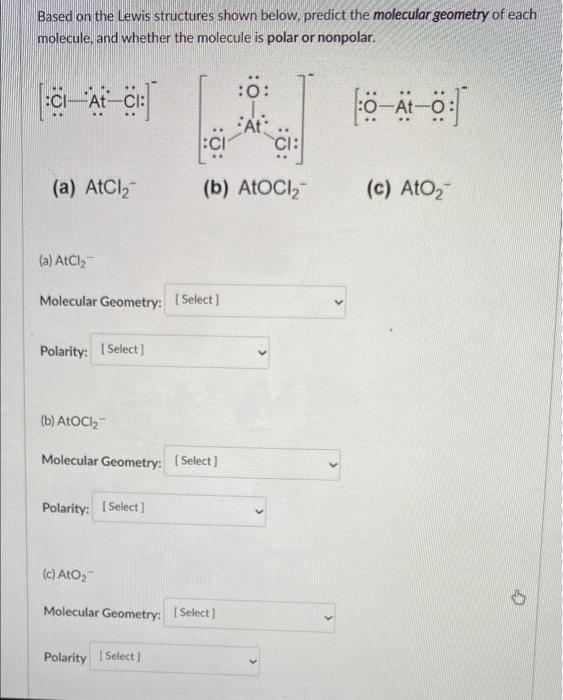
(7 pts) WRITTEN: ENTER ANSWER ON WRITTEN TEMPLATE Consider the molecule PSBr. P is the central atom in the molecule, and the 5 and Br atoms are all terminal atoms. Draw the correct Lewis structure for this molecule. Be sure to draw all lone pairs, and label all non-zero formal charges on each atom. If multiple resonance structures are possible, draw the structure that minimizes formal charges on the atoms. Circle the ONE structure you would like to be graded. Only the structure that is CIRCLED will be graded. Based on the Lewis structures shown below, predict the molecular geometry of each molecule, and whether the molecule is polar or nonpolar. |:CIA CI: (a) AtCl (a) AtCl Molecular Geometry: [Select] Polarity: [Select] (b) AtoCl Molecular Geometry: [Select] Polarity: [Select] (b) AtoCl (c) AtO Molecular Geometry: [Select] Polarity [Select] :0: At CI .. 0-At-0: (c) AtO
Step by Step Solution
★★★★★
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


