Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7. Techniques of protein purification a. Why is SDS omitted when proteins need to undergo isoelectric focusing? b. A series of proteins with a
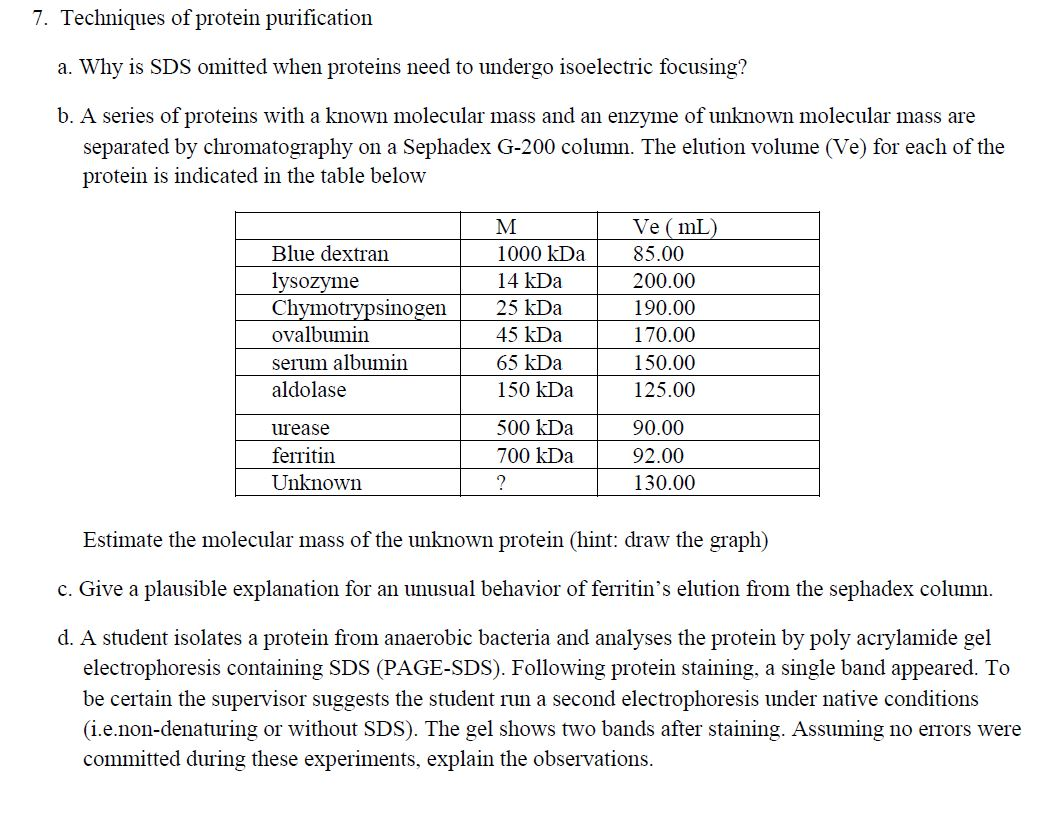
7. Techniques of protein purification a. Why is SDS omitted when proteins need to undergo isoelectric focusing? b. A series of proteins with a known molecular mass and an enzyme of unknown molecular mass are separated by chromatography on a Sephadex G-200 column. The elution volume (Ve) for each of the protein is indicated in the table below Blue dextran lysozyme Chymotrypsinogen ovalbumin serum albumin aldolase urease ferritin Unknown M 1000 kDa 14 kDa 25 kDa 45 kDa 65 kDa 150 kDa 500 kDa 700 kDa ? Ve (mL) 85.00 200.00 190.00 170.00 150.00 125.00 90.00 92.00 130.00 Estimate the molecular mass of the unknown protein (hint: draw the graph) c. Give a plausible explanation for an unusual behavior of ferritin's elution from the sephadex column. d. A student isolates a protein from anaerobic bacteria and analyses the protein by poly acrylamide gel electrophoresis containing SDS (PAGE-SDS). Following protein staining, a single band appeared. To be certain the supervisor suggests the student run a second electrophoresis under native conditions (i.e.non-denaturing or without SDS). The gel shows two bands after staining. Assuming no errors were committed during these experiments, explain the observations.
Step by Step Solution
★★★★★
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Answer 7a Isoelectric point is that pH when the proteins net charge is zero ie in electrically neutral state The purpose of the isoelectric focusing i...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


