Question
(7) The formation of boron trinitrate can be done by the reaction of: B:Broe) + HNOS(a4) B(NO:)s(ac) + HBr(aq) a) How many grams of
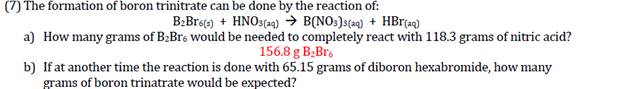
(7) The formation of boron trinitrate can be done by the reaction of: B:Broe) + HNOS(a4) B(NO:)s(ac) + HBr(aq) a) How many grams of B2Bre would be needed to completely react with 118.3 grams of nitric acid? 156.8 g B;Br. b) If at another time the reaction is done with 65.15 grams of diboron hexabromide, how many grams of boron trinatrate would be expected?
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Oate Poge Na Given reaction H Briag first we balance the chemical equation B BYt 6HNO3 ag 2 BNOgt H8...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Data Communications and Networking
Authors: Behrouz A. Forouzan
5th edition
73376221, 978-0073376226
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



