Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7) Which of the following 100.0g samples contains the greatest number of atoms? [3] A. Potassium B. Iodine C. Cobalt D. Silver 1) Which of

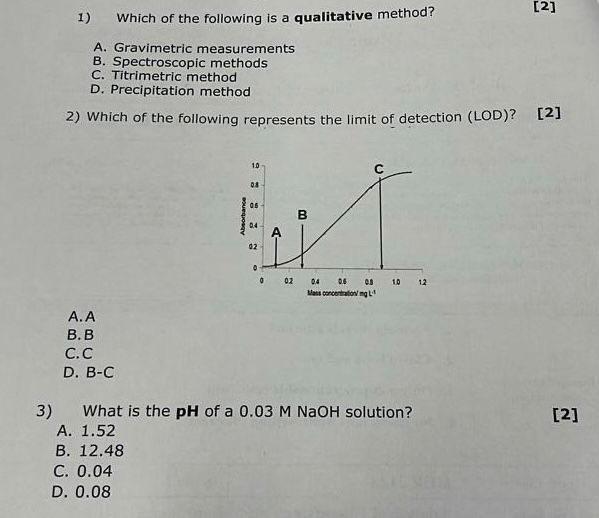

 7) Which of the following 100.0g samples contains the greatest number of atoms? [3] A. Potassium B. Iodine C. Cobalt D. Silver 1) Which of the following is a qualitative method? A. Gravimetric measurements B. Spectroscopic methods C. Titrimetric method D. Precipitation method 2) Which of the following represents the limit of detection (LOD)? [2] A. A B. B C. C D. BC 3) What is the pH of a 0.03MNaOH solution? A. 1.52 B. 12.48 C. 0.04 D. 0.08 4) The OHconcentration in a 1.0103MBa(OH)2 solution is A. 0.50103M B. 1.0103M C. 2.0103M D. 1.0102M 5) The answer to the calculation 87.83.00.080065 should have how n significant figures? A. 3 B. 6 C. 2 D. 4 6) Identify the conjugate Base of H2CO3. A. H2O B. HCO3 C. CO32 D. CO2 7) 15ml of 0.1MHCl is titrated with 0.2MNaOH. Calculate the pH after the addition of 5ml of titrant [2] A. 4 B. 3 C. 1.6 D. 12.4 8) According to Acid-Base theories, which one of the below is a Base [1] A. Electron pain acceptor B. Proton donor C. Proton acceptor D. All of the above
7) Which of the following 100.0g samples contains the greatest number of atoms? [3] A. Potassium B. Iodine C. Cobalt D. Silver 1) Which of the following is a qualitative method? A. Gravimetric measurements B. Spectroscopic methods C. Titrimetric method D. Precipitation method 2) Which of the following represents the limit of detection (LOD)? [2] A. A B. B C. C D. BC 3) What is the pH of a 0.03MNaOH solution? A. 1.52 B. 12.48 C. 0.04 D. 0.08 4) The OHconcentration in a 1.0103MBa(OH)2 solution is A. 0.50103M B. 1.0103M C. 2.0103M D. 1.0102M 5) The answer to the calculation 87.83.00.080065 should have how n significant figures? A. 3 B. 6 C. 2 D. 4 6) Identify the conjugate Base of H2CO3. A. H2O B. HCO3 C. CO32 D. CO2 7) 15ml of 0.1MHCl is titrated with 0.2MNaOH. Calculate the pH after the addition of 5ml of titrant [2] A. 4 B. 3 C. 1.6 D. 12.4 8) According to Acid-Base theories, which one of the below is a Base [1] A. Electron pain acceptor B. Proton donor C. Proton acceptor D. All of the above




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


