Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7.14 Indicate whether each of the following statements about a process in a closed system with a constant volume is always true, always false,
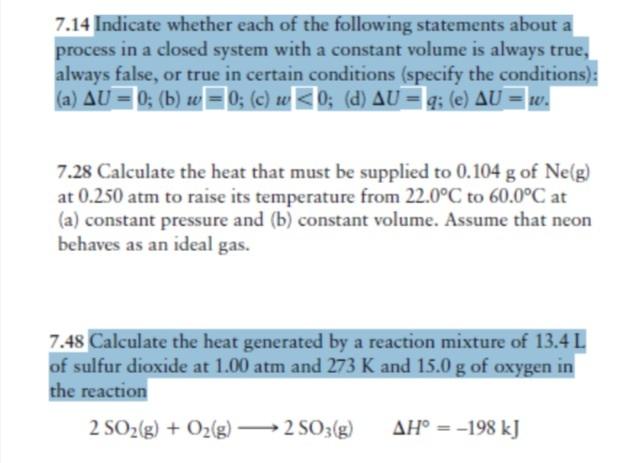
7.14 Indicate whether each of the following statements about a process in a closed system with a constant volume is always true, always false, or true in certain conditions (specify the conditions): (a) AU = 0; (b) w = 0; (c) w 0; (d) AU = q; (e) AU = w. 7.28 Calculate the heat that must be supplied to 0.104 g of Ne(g) at 0.250 atm to raise its temperature from 22.0C to 60.0C at (a) constant pressure and (b) constant volume. Assume that neon behaves as an ideal gas. 7.48 Calculate the heat generated by a reaction mixture of 13.4 L of sulfur dioxide at 1.00 atm and 273 K and 15.0 g of oxygen in the reaction 2 SO(g) + O(g) 2SO3(g) AH = -198 kJ
Step by Step Solution
★★★★★
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
714 We know As in a closed system with const volume T is definitely going ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


