Answered step by step
Verified Expert Solution
Question
1 Approved Answer
73.- (exam jan'09) In a 10L reactor the elementary reversible reaction CAB takes place in liquid phase at constant temperature. In order to determine whether
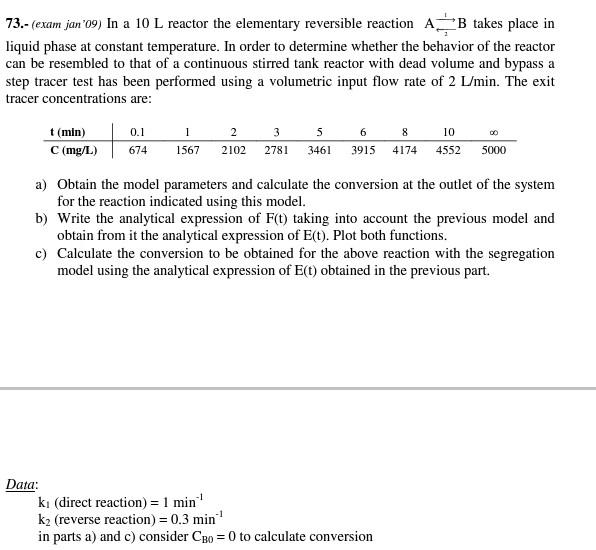
73.- (exam jan'09) In a 10L reactor the elementary reversible reaction CAB takes place in liquid phase at constant temperature. In order to determine whether the behavior of the reactor can be resembled to that of a continuous stirred tank reactor with dead volume and bypass a step tracer test has been performed using a volumetric input flow rate of 2L/min. The exit tracer concentrations are: a) Obtain the model parameters and calculate the conversion at the outlet of the system for the reaction indicated using this model. b) Write the analytical expression of F(t) taking into account the previous model and obtain from it the analytical expression of E(t). Plot both functions. c) Calculate the conversion to be obtained for the above reaction with the segregation model using the analytical expression of E(t) obtained in the previous part. Data: k1( direct reaction )=1min1 k2 (reverse reaction) =0.3min1 in parts a) and c) consider CB0=0 to calculate conversion
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


