Answered step by step
Verified Expert Solution
Question
1 Approved Answer
78.- (exam jan'1l) In a reactor of volume V=10L you want to carry out the reaction 2AB in liquid phase at constant temperature. The feeding
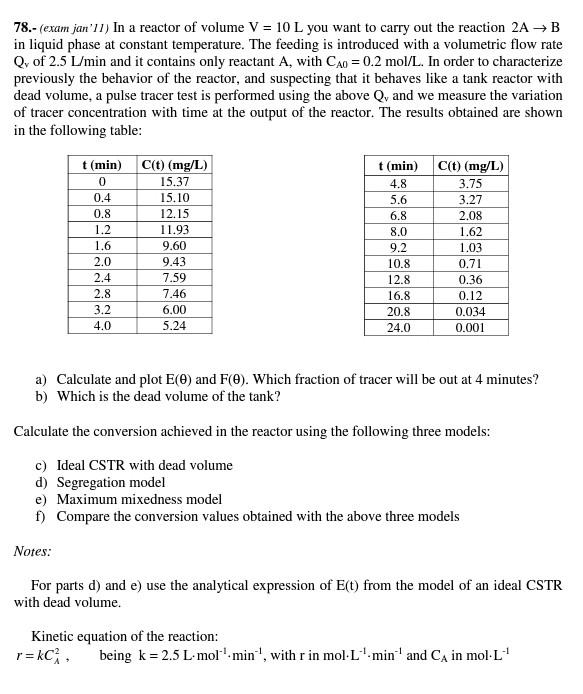
78.- (exam jan'1l) In a reactor of volume V=10L you want to carry out the reaction 2AB in liquid phase at constant temperature. The feeding is introduced with a volumetric flow rate Qv of 2.5L/min and it contains only reactant A, with CA0=0.2mol/L. In order to characterize previously the behavior of the reactor, and suspecting that it behaves like a tank reactor with dead volume, a pulse tracer test is performed using the above Qv and we measure the variation of tracer concentration with time at the output of the reactor. The results obtained are shown in the following table: a) Calculate and plot E() and F(). Which fraction of tracer will be out at 4 minutes? b) Which is the dead volume of the tank? Calculate the conversion achieved in the reactor using the following three models: c) Ideal CSTR with dead volume d) Segregation model e) Maximum mixedness model f) Compare the conversion values obtained with the above three models Notes: For parts d) and e) use the analytical expression of E(t) from the model of an ideal CSTR with dead volume. Kinetic equation of the reaction: r=kCA2, being k=2.5Lmol1min1, with r in molL1min1 and CA in molL1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


