Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7,9,11 and 13 7. Write the chemical equation for nitric acid digestion of (a) ADU; (b) MDU; (c) SDU; and (d) triuranium octoxide. 8. Look
7,9,11 and 13 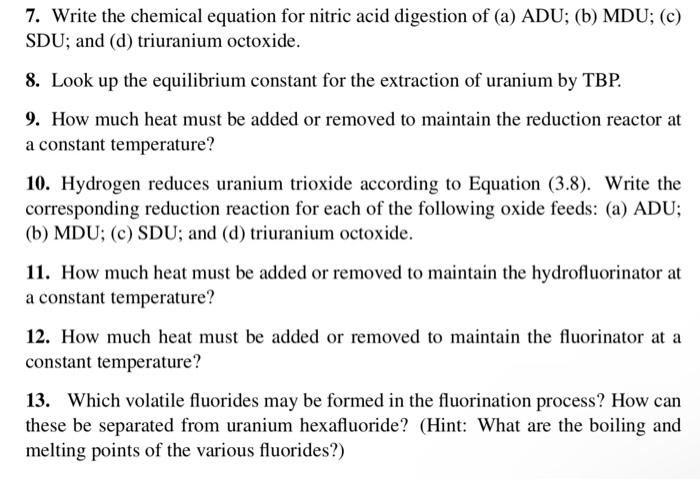
7. Write the chemical equation for nitric acid digestion of (a) ADU; (b) MDU; (c) SDU; and (d) triuranium octoxide. 8. Look up the equilibrium constant for the extraction of uranium by TBP. 9. How much heat must be added or removed to maintain the reduction reactor at a constant temperature? 10. Hydrogen reduces uranium trioxide according to Equation (3.8). Write the corresponding reduction reaction for each of the following oxide feeds: (a) ADU; (b) MDU; (c) SDU; and (d) triuranium octoxide. 11. How much heat must be added or removed to maintain the hydrofluorinator at a constant temperature? 12. How much heat must be added or removed to maintain the fluorinator at a constant temperature? 13. Which volatile fluorides may be formed in the fluorination process? How can these be separated from uranium hexafluoride? (Hint: What are the boiling and melting points of the various fluorides?) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


