Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8. 12. The major resonance contributor to the resonance hybrid will have the greatest separation of formal charges. True False 19. Cis-trans isomerism is possible
8.
12. The major resonance contributor to the resonance hybrid will have the greatest separation of formal charges.
True
False
19. Cis-trans isomerism is possible only in the case of:
A. CH 2=CHBr
B. Br 2C=CHF
C. F2C=CF2
D. CF 2=CBr 2
E. BrCF=CFBr
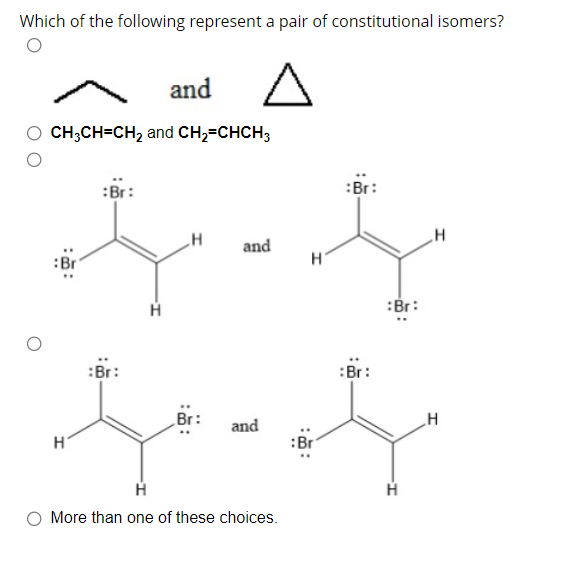
Which of the following represent a pair of constitutional isomers? and CH3CH=CH2 and CH2=CHCH3 and and More than one of these choices
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


