Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8 of 17 This Quiz: 17 A certain element has a half life of 4.5 billion years. a. You find a rock containing a mixture
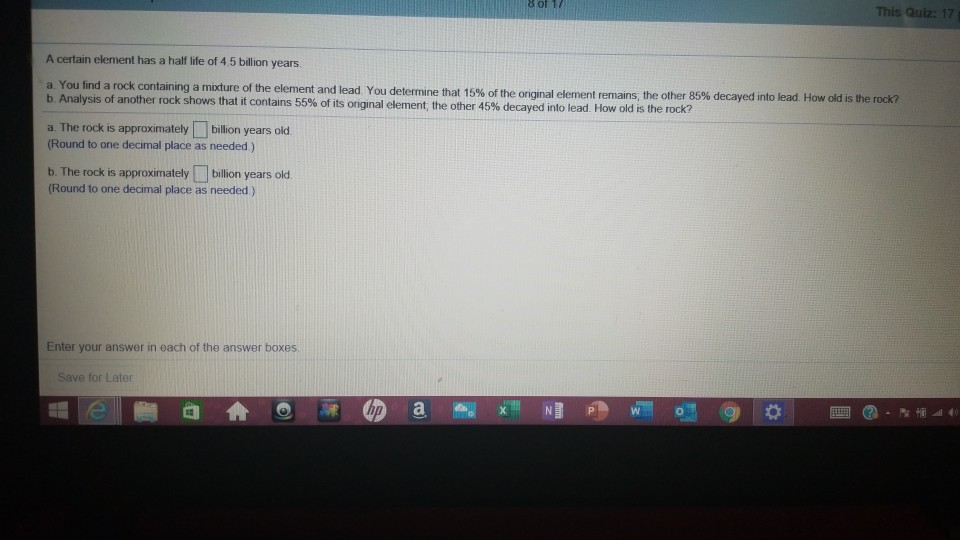
8 of 17 This Quiz: 17 A certain element has a half life of 4.5 billion years. a. You find a rock containing a mixture of the element and lead. You determine that 15% of the original element remains; the other 85% decayed into lead. How old is the rock? b. Analysis of another rock shows that it contains 55% of its original element; the other 45% decayed into lead. How old is the rock? a. The rock is approximately billion vears old (Round to one decimal place as needed) b. The rock is approximately billion years old. (Round to one decimal place as needed) Enter your answer in each of the answer boxes Save for Later hn W
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


