8. sulfur tetrachloride 9. azide ion, N, 10. beryllium dichloride 11. boron trifluoride #valence electrons electron-domain geometry molecular geometry bond angles #valence electrons electron-domain

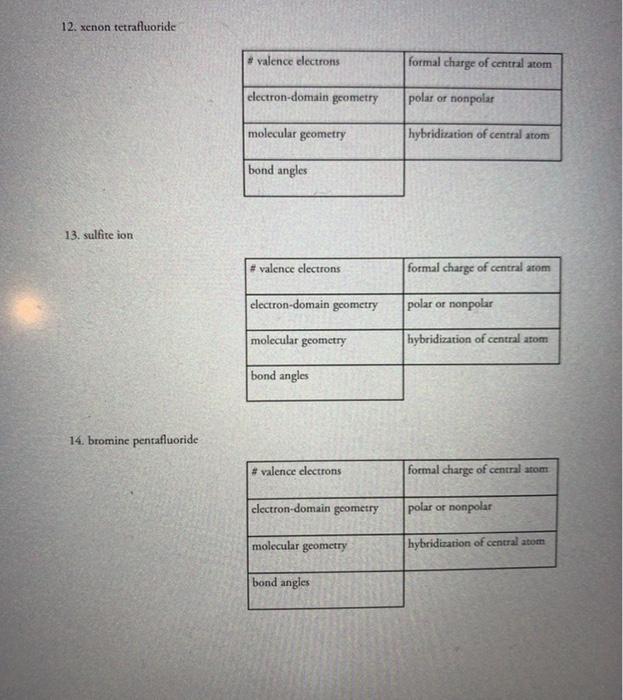
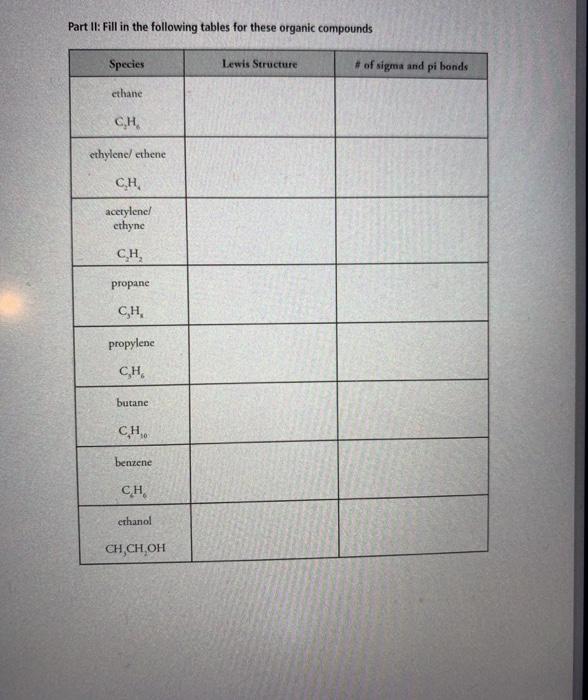

8. sulfur tetrachloride 9. azide ion, N, 10. beryllium dichloride 11. boron trifluoride #valence electrons electron-domain geometry molecular geometry bond angles #valence electrons electron-domain molecular geometry bond angles geometry #valence electrons electron-domain geometry molecular geometry bond angles #valence electrons electron-domain geometry molecular geometry bond angles formal charge of central atom polar or nonpolar hybridization of central atom formal charge of central atom polar or nonpolar hybridization of central atom formal charge of central atom polar or nonpolar hybridization of central atom formal charge of central atom polar or nonpolar hybridization of central atom 12. xenon tetrafluoride 13. sulfite ion 14. bromine pentafluoride #valence electrons electron-domain geometry molecular geometry bond angles #valence electrons electron-domain geometry molecular geometry bond angles #valence electrons electron-domain geometry molecular geometry bond angles formal charge of central atom polar or nonpolar hybridization of central atom formal charge of central atom polar or nonpolar hybridization of central atom formal charge of central atom polar or nonpolar hybridization of central atom Part II: Fill in the following tables for these organic compounds Species ethane C.H ethylene/ ethene C.H. acetylene/ ethyne CH propane C,H, propylene C,H, butane CH, benzene CH ethanol CH,CH,OH Lewis Structure # of sigma and pi bonds Part III: Fill in the following tables for these ions and acids Species acetate ion hint: CH,CO, acetic acid hypochlorite ion chlorite ion hypochlorous acid chlorous acid sulfate ion phosphate ion Lewis Structure
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
1 Sulfur Tetrachloride Valence electrons 34 Formal charge of central atom 0 Electrondomain geometry ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


