Question
Using the Van der Waals equation of state, what is the change in internal energy induced by the isothermal expansion of 1 mol propane
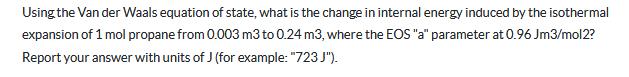
Using the Van der Waals equation of state, what is the change in internal energy induced by the isothermal expansion of 1 mol propane from 0.003 m3 to 0.24 m3, where the EOS "a" parameter at 0.96 Jm3/mol2? Report your answer with units of J (for example: "723 J").
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Answer The Van der Waals equation of state can be written as P nRTV aV2 bV3 where P is the pressure ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Horngrens Financial and Managerial Accounting
Authors: Tracie L. Nobles, Brenda L. Mattison, Ella Mae Matsumura
5th edition
9780133851281, 013385129x, 9780134077321, 133866297, 133851281, 9780133851298, 134077326, 978-0133866292
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



