Answered step by step
Verified Expert Solution
Question
1 Approved Answer
85. Predict the products in the given reaction. CHO (1) C1 C1 50% KOH -CHOH COO C1 (2) O CH,OH -COO OH OH -CHOH
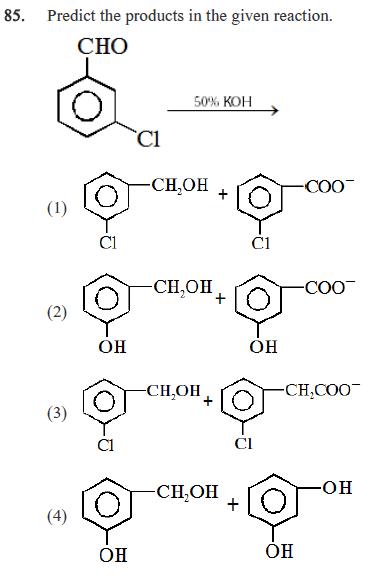
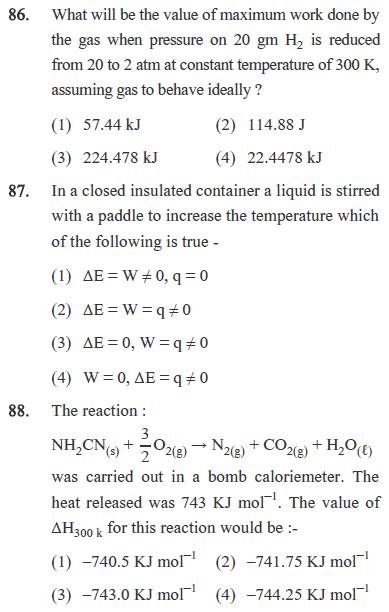
85. Predict the products in the given reaction. CHO (1) C1 C1 50% KOH -CHOH COO C1 (2) O CH,OH -COO OH OH -CHOH -CH,COO (3) (4) OH -CHOH -OH + OH 86. What will be the value of maximum work done by the gas when pressure on 20 gm H is reduced from 20 to 2 atm at constant temperature of 300 K, assuming gas to behave ideally? (1) 57.44 kJ (2) 114.88 J (3) 224.478 kJ (4) 22.4478 kJ 87. In a closed insulated container a liquid is stirred with a paddle to increase the temperature which of the following is true - (1) AE=W0, q=0 (2) AE W 90 (3) AE 0, Wq0 (4) W 0, AE=q=0 88. The reaction: 3 NHCN (s) + O2(g) N2(g) + CO2(g) + HO(f) 2 was carried out in a bomb caloriemeter. The heat released was 743 KJ mol. The value of AH 300k for this reaction would be :- (1)-740.5 KJ mol (2) -741.75 KJ mol (3) -743.0 KJ mol (4) -744.25 KJ mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1
1 CH2OH COO C...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


