Question
8.71mL of a 4.00M HIO3(aq) solution is added to an ice-water mixture, and the freezing points were collected before and after addition of HIO3(aq).
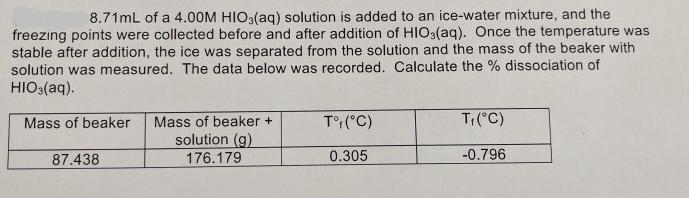
8.71mL of a 4.00M HIO3(aq) solution is added to an ice-water mixture, and the freezing points were collected before and after addition of HIO3(aq). Once the temperature was stable after addition, the ice was separated from the solution and the mass of the beaker with solution was measured. The data below was recorded. Calculate the % dissociation of HIO3(aq). Mass of beaker Mass of beaker + T: (C) Ti(C) solution (g) 87.438 176.179 0.305 -0.796
Step by Step Solution
3.29 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
delta Tf i...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: John D. Cutnell, Kenneth W. Johnson
9th edition
470879564, 1118424840, 470879521, 9780470879566, 9781118424841, 978-0470879528
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



