Question
The reaction 2NOBr 2NO+Br2 exhibits the rate law =[NOB:] - A[NOBr] At Rate = where k 1.0 x 10-5 Ms at 25C. This reaction
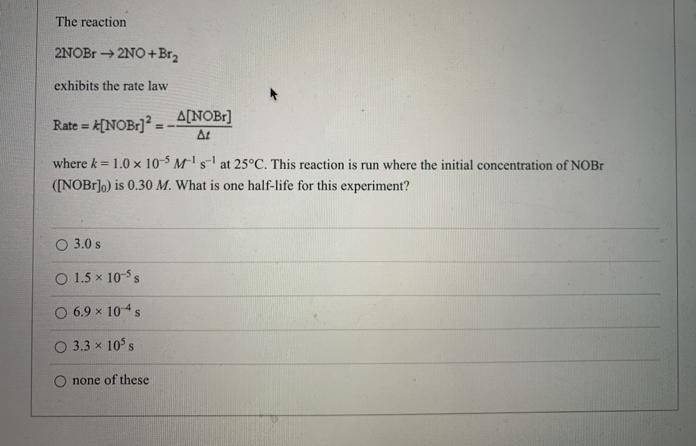
The reaction 2NOBr 2NO+Br2 exhibits the rate law =[NOB:] - A[NOBr] At Rate = where k 1.0 x 10-5 Ms at 25C. This reaction is run where the initial concentration of NOBR ([NOBIJ,) is 0.30 M. What is one half-life for this experiment? O 3.0 s O 1.5 x 10-5s O 6.9 x 104s 3.3 x 10 s none of these
Step by Step Solution
3.51 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
zivon K NOBZ oNOBL when ot KCNOBA Integrating dENo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



