Answered step by step
Verified Expert Solution
Question
1 Approved Answer
9. The ester of an organic base is hydrolyzed in a CSTR. The rate of this irre- versible reaction is first-order in each reactant.
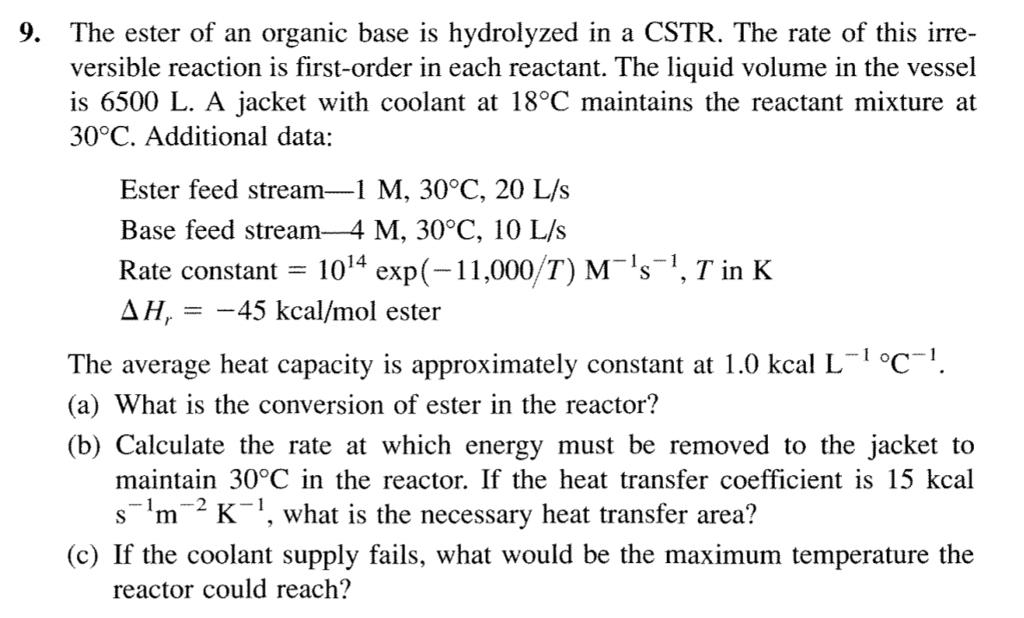
9. The ester of an organic base is hydrolyzed in a CSTR. The rate of this irre- versible reaction is first-order in each reactant. The liquid volume in the vessel is 6500 L. A jacket with coolant at 18C maintains the reactant mixture at 30C. Additional data: Ester feed stream-1 M, 30C, 20 L/s Base feed stream-4 M, 30C, 10 L/s Rate constant = 104 exp(-11,000/T) Ms, T in K AH, = 45 kcal/mol ester The average heat capacity is approximately constant at 1.0 kcal L- C. (a) What is the conversion of ester in the reactor? (b) Calculate the rate at which energy must be removed to the jacket to maintain 30C in the reactor. If the heat transfer coefficient is 15 kcal sm2K, what is the necessary heat transfer area? (c) If the coolant supply fails, what would be the maximum temperature the reactor could reach?
Step by Step Solution
★★★★★
3.39 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
given Ester Bace IVGROOL Coolant S n1 1st order 18C Temp Traix 30 C I Tin To 30C Ester f...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


