Question
A 0.06 g of the NaCl sample (FM: 58.44) was treated with 15.00 mL of standard 0.120 M AGNO, (FM: 169.872) solution. The excess
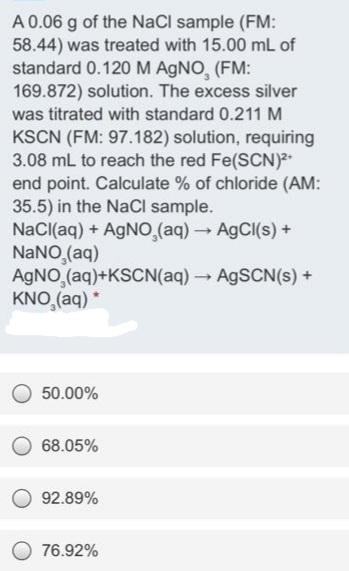
A 0.06 g of the NaCl sample (FM: 58.44) was treated with 15.00 mL of standard 0.120 M AGNO, (FM: 169.872) solution. The excess silver was titrated with standard 0.211 M KSCN (FM: 97.182) solution, requiring 3.08 mL to reach the red Fe(SCN) end point. Calculate % of chloride (AM: 35.5) in the NaCl sample. NaCl(aq) + AGNO (aq)A9CI(s)+ NANO (aq) AGNO,(aq)+KSCN(aq) AGSCN(s) + KNO,(aq) * 50.00% 68.05% 92.89% 76.92%
Step by Step Solution
3.33 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Ans 6805 Explanation From the given reaction AgNO 3 KSCN AgSCN KNO 3 we can see 1 AgNO 3 requires 1 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



