Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 0.1070 g rock sample containing an unknown amount of Ca was dissolved in HCl and diluted to 100.0 mL. Aliquots of 20.00 mL
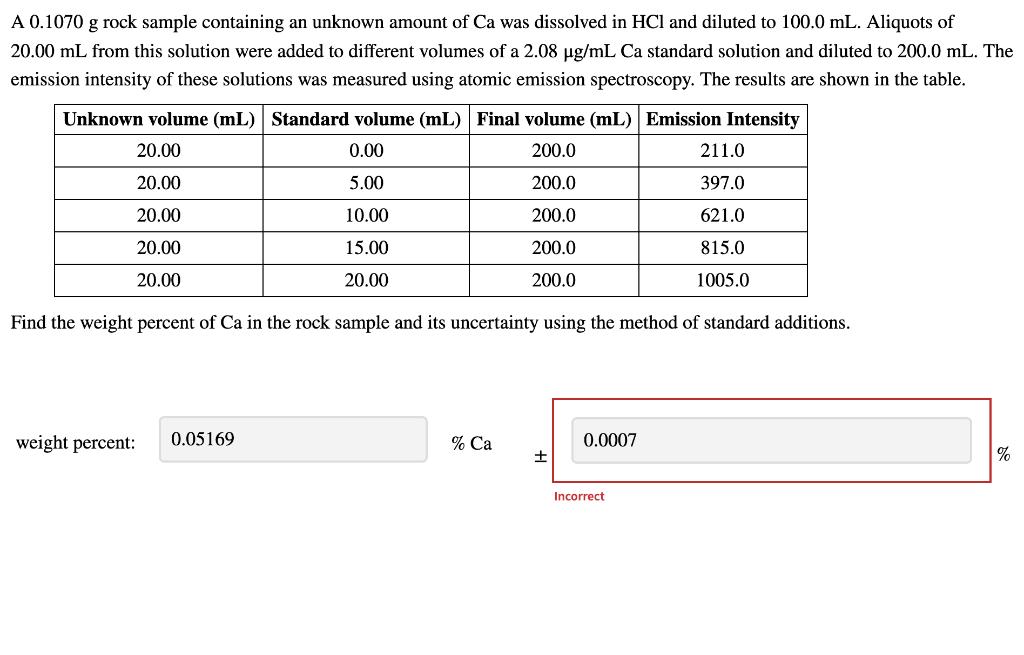
A 0.1070 g rock sample containing an unknown amount of Ca was dissolved in HCl and diluted to 100.0 mL. Aliquots of 20.00 mL from this solution were added to different volumes of a 2.08 ug/mL Ca standard solution and diluted to 200.0 mL. The emission intensity of these solutions was measured using atomic emission spectroscopy. The results are shown in the table. Unknown volume (mL) Standard volume (mL) Final volume (mL) Emission Intensity 20.00 0.00 200.0 211.0 20.00 5.00 200.0 397.0 20.00 10.00 200.0 621.0 20.00 15.00 200.0 815.0 20.00 20.00 200.0 1005.0 Find the weight percent of Ca in the rock sample and its uncertainty using the method of standard additions. weight percent: 0.05169 % Ca 0.0007 % Incorrect
Step by Step Solution
★★★★★
3.51 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636ae4b0af8c9_234378.pdf
180 KBs PDF File
636ae4b0af8c9_234378.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


