Question
A 0.3 kg of metal bar initially at 1200 K is immersed in a closed tank containing 9 kg of water initially at 300
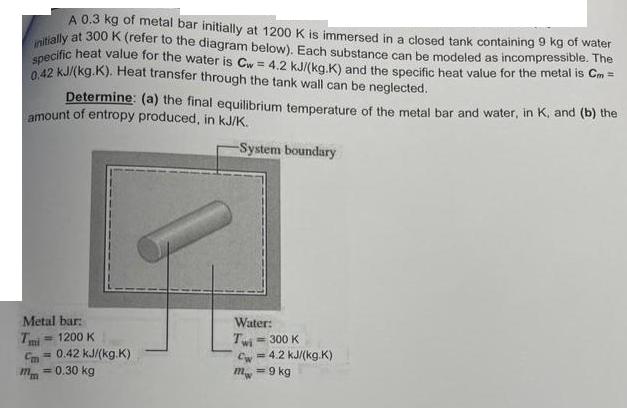
A 0.3 kg of metal bar initially at 1200 K is immersed in a closed tank containing 9 kg of water initially at 300 K (refer to the diagram below). Each substance can be modeled as incompressible. The specific heat value for the water is Cw = 4.2 kJ/(kg.K) and the specific heat value for the metal is Cm= 0.42 kJ/(kg.K). Heat transfer through the tank wall can be neglected. Determine: (a) the final equilibrium temperature of the metal bar and water, in K, and (b) the amount of entropy produced, in kJ/K. -System boundary Metal bar: Tmi Cm = 0.42 kJ/(kg.K) mm = 0.30 kg 1200 K Water: Twi
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Problem A 03 kg metal bar initially at 1200 K is immersed in a closed tank containing 9 kg of water ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Chemical Engineering Thermodynamics
Authors: Kevin D. Dahm, Donald P. Visco
1st Edition
1111580707, 978-1111580704
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



