Question
A 0.5530 kg ice cube at -12.40 C is placed inside a rigid, thermally isolated chamber containing 6.910 moles of steam at 365.0C. Later,
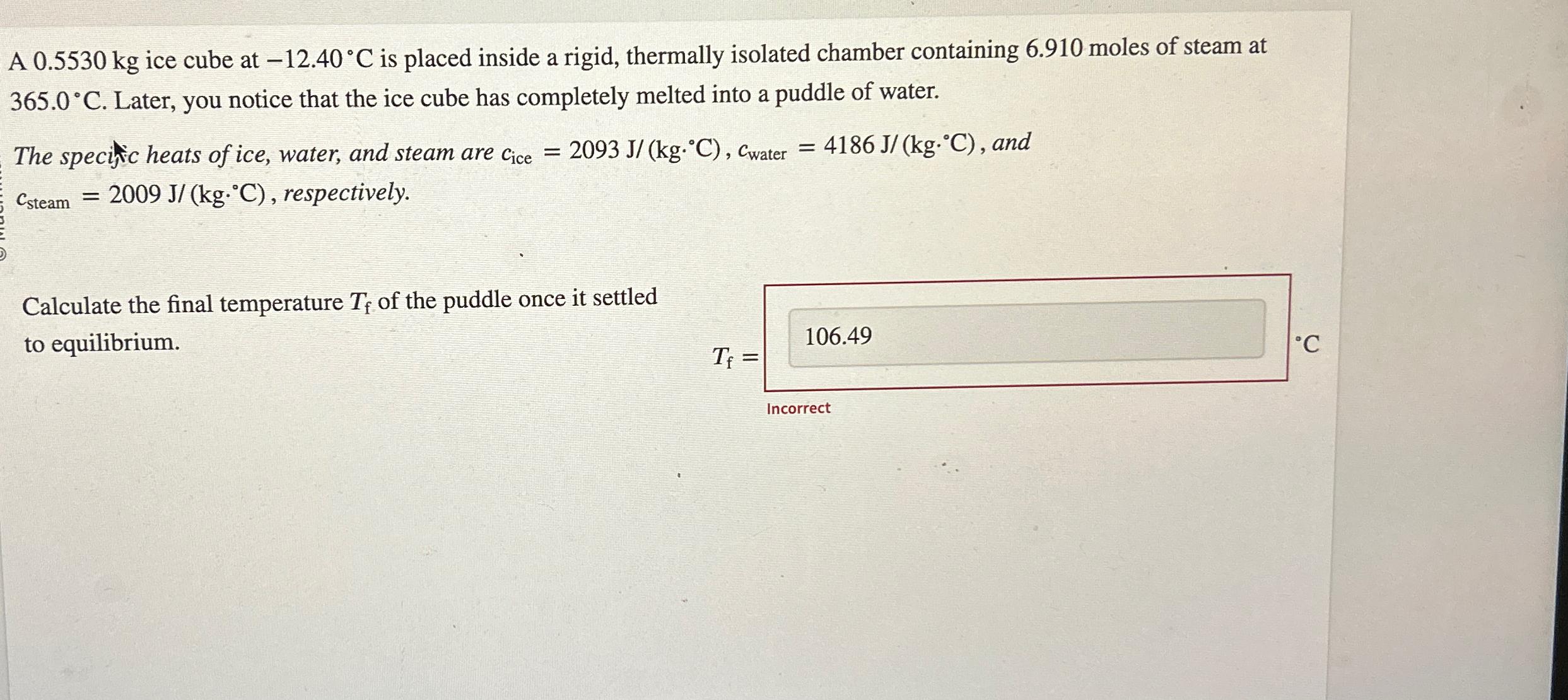
A 0.5530 kg ice cube at -12.40 C is placed inside a rigid, thermally isolated chamber containing 6.910 moles of steam at 365.0C. Later, you notice that the ice cube has completely melted into a puddle of water. The specific heats of ice, water, and steam are Cice = 2093 J/(kg.C), Cwater = 4186 J/(kg.C), and Csteam = 2009 J/(kg.C), respectively. Calculate the final temperature Tf of the puddle once it settled to equilibrium. 106.49 T = Incorrect C
Step by Step Solution
There are 3 Steps involved in it
Step: 1
A nice thermodynamics problem Lets break it down step by step Given Mass of the ice cube 05530 kg In...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
University Physics with Modern Physics
Authors: Hugh D. Young, Roger A. Freedman, A. Lewis Ford
13th edition
321696867, 978-0321696861
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



